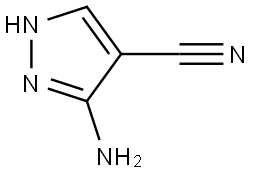
3-Amino-4-pyrazolecarbonitrile synthesis
- Product Name:3-Amino-4-pyrazolecarbonitrile
- CAS Number:16617-46-2
- Molecular formula:C4H4N4
- Molecular Weight:108.1
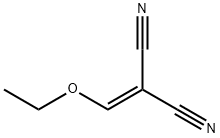
123-06-8

16617-46-2
The general procedure for the synthesis of 3-amino-4-cyanopyrazole from ethoxymethylene malononitrile was as follows: hydrazine hydrate (5 g, 0.156 mol) was slowly added dropwise to an ethanol solution containing 2-(ethoxymethylene)malononitrile (8.64 g, 0.070 mol), and the reaction mixture was subsequently heated for 30 min on a steam bath. A white precipitate gradually precipitated during the reaction and the reaction mixture was transferred to a refrigerator and left overnight to complete crystallization. Subsequently, the white solid product was collected by filtration and washed with a small amount of pre-cooled ethanol to finally obtain the target compound 5-amino-1H-pyrazole-4-carbonitrile (1) as white crystals in 86% (3.05 g) yield; the melting point was 168-170 °C (literature value: 169-170 °C) [33].
122-51-0
514 suppliers
$10.00/5ml

109-77-3
453 suppliers
$10.00/5g

16617-46-2
502 suppliers
$6.00/5g
Yield:16617-46-2 72%
Reaction Conditions:
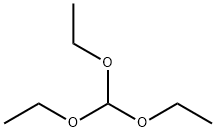
Stage #1:orthoformic acid triethyl ester;malononitrile with acetic anhydride at 115; for 2 h;
Stage #2: with hydrazine hydrate at 18 - 30; for 18.6667 h;
Steps:
Amino-1H-pyrazole-4-carbonitrile
Add malononitrile (16.5 g, 0.25 mol), triethyl orthoformate (38 g, 0.29 mol) and acetic anhydride (55 g,0.54 mol) to a 1 L three-necked flask, slowly heat to 115 °C, reflux reaction for 2 h .The temperature of the mixture was lowered to 20 °C,hydrazine hydrate (15.5 g, 0.31 mol) was added dropwiseover 40minutes, and the temperature was maintained at 18-22 °C.The reaction was then carried out at 20-30 ° C for 18 h.Thereaction wasmonitoredbyTLC, neutralized with a sodium hydroxide aqueous solution (36 g of sodium hydroxide in 72 ml of water) at 25 °C to obtain a prize mixture, and the mixture washeated and azeotropically distilled to collect 45 ml fractions.This fraction was cooled at 0 to 5 ° C for 1.5 h, and a pale brown solid was collected,rinsedwithcold water and dried to give product (19.2 g, 72% yield).
References:
Jiangnan University;Tang Chunlei;Cao Kai;Zhao Hui;Hu Xiaoxia;Fan Weizheng;Feng Bonian;Liao Hanyue CN108484613, 2018, A Location in patent:Paragraph 0044; 0045; 0046

123-06-8
350 suppliers
$14.14/1gm:

16617-46-2
502 suppliers
$6.00/5g
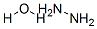
7803-57-8
0 suppliers
$20.10/50g

123-06-8
350 suppliers
$14.14/1gm:

16617-46-2
502 suppliers
$6.00/5g
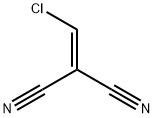
10472-09-0
2 suppliers
inquiry

16617-46-2
502 suppliers
$6.00/5g
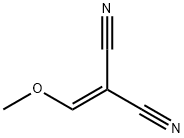
672-81-1
24 suppliers
inquiry

16617-46-2
502 suppliers
$6.00/5g