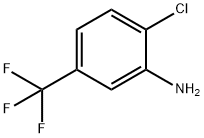
3-Amino-4-chlorobenzotrifluoride synthesis
- Product Name:3-Amino-4-chlorobenzotrifluoride
- CAS Number:121-50-6
- Molecular formula:C7H5ClF3N
- Molecular Weight:195.57
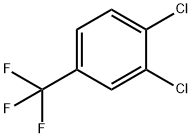
328-84-7
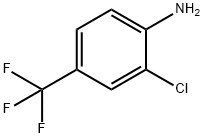
39885-50-2

121-50-6
Example 1: N-methylpyrrolidone (1050 mL) was added to an autoclave with 102 g of anhydrous activated potassium fluoride followed by 377 g of 3,4-dichlorobenzotrifluoride. At ambient temperature, 158 g of ammonia was vented from a pressure tank into the reactor. The reaction mixture was heated to 245-250 °C over a period of 2 h, at which time the reactor pressure reached 30-32 kg/cm2. excess ammonia gas was continued to be passed from the pressure tank to maintain the reactor pressure at 38-40 kg/cm2 at a liquid temperature of 245-250 °C. The reaction was maintained at this temperature and pressure condition for 8 h. The reaction was completed with the addition of 377 g of potassium fluoride, followed by the addition of 377 g of 3,4-dichlorobenzotrifluoride. Upon completion of the reaction, the mixture was cooled to ambient temperature and the unreacted ammonia gas was drained and recovered. The reaction mixture was filtered and the products were separated by fractional distillation to give 2-chloro-4-trifluoromethylaniline (77% yield) and 2-chloro-5-trifluoromethylaniline (13% yield) based on 3,4-dichlorobenzotrifluoride consumption calculations.

328-84-7
385 suppliers
$5.00/5g

39885-50-2
272 suppliers
$10.00/10 g

121-50-6
343 suppliers
$5.00/5g
Yield:121-50-6 13% ,39885-50-2 77%
Reaction Conditions:
with potassium fluoride;ammonia in 1-methyl-pyrrolidin-2-one at 245 - 250; under 22067.2 - 29422.9 Torr; for 10 h;Autoclave;
Steps:
1
Example- 1; N-methylpyrrolidone (1050 ml) was charged in an autoclave along with 102 g anhydrous activated potassium fluoride, 377 g 3,4- dichlorobenzotrifluoride was added. Ammonia (158 g) gas was passed in the reactor from the pressure pot at ambient temperature. The content of the reactor was heated to 245-250°C over a period of 2 hours to get reactor pressure of 30-32 kg/cm2. Excess NH3 was fed from the pressure pot to maintain the reactor pressure at 38-40 kg/cm at 245-250°C liquid temperature. Reaction mixture was maintained at 245-250°C and at 38-40 kg/cm2 pressure for further 8 hours. Reaction mixture was cooled to ambient temperature and NH3 was vented and recovered. Reaction mixture was filtered and on fractionation gave 77% yield of 2-chloro-4- trifluoromethylaniline and 13% yield of 2-chloro-5-trifluoro methyl aniline based on consumed 3,4-dichlorobenzotrifluoride.
References:
GHARDA, Keki Hormusji WO2011/107998, 2011, A1 Location in patent:Page/Page column 17
![1-[2-CHLORO-5-(TRIFLUOROMETHYL)PHENYL]HYDRAZINE](/CAS/GIF/1869-22-3.gif)
1869-22-3
71 suppliers
inquiry
![1-[2-CHLORO-4-(TRIFLUOROMETHYL)PHENYL]HYDRAZINE](/CAS/GIF/86398-98-3.gif)
86398-98-3
55 suppliers
inquiry

39885-50-2
272 suppliers
$10.00/10 g

121-50-6
343 suppliers
$5.00/5g
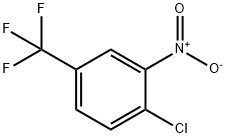
121-17-5
32 suppliers
$19.00/250g

121-50-6
343 suppliers
$5.00/5g

7732-18-5
507 suppliers
$12.69/100ml

121-17-5
32 suppliers
$19.00/250g

121-50-6
343 suppliers
$5.00/5g
98-56-6
410 suppliers
$11.00/25g

121-50-6
343 suppliers
$5.00/5g