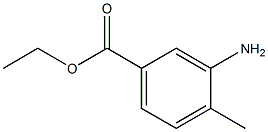
3-AMINO-4-METHYLBENZOIC ACID ETHYL ESTER synthesis
- Product Name:3-AMINO-4-METHYLBENZOIC ACID ETHYL ESTER
- CAS Number:917391-29-8
- Molecular formula:C10H13NO2
- Molecular Weight:179.2157
![1H-IMidazole, 4-Methyl-1-[3-nitro-5-(trifluoroMethyl)phenyl]-](/CAS/GIF/916975-92-3.gif)
916975-92-3
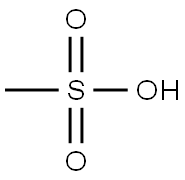
75-75-2

917391-29-8
Example 12 Synthesis of 4-methyl-1-(3-nitro-5-(trifluoromethyl)phenyl)-1H-imidazole methanesulfonate (XXIX): the crude 4-methyl-1-(3-nitro-5-(trifluoromethyl)phenyl)-1H-imidazole (IX) (1.85 g, 6 mmol, HPLC purity 88 area%) was dissolved in ethyl acetate (6 mL) and the temperature was maintained at about 50 °C. Methanesulfonic acid (0.397 mL, 6 mmol) was slowly added to the stirred black solution. Upon completion of the addition, a bright solid was observed to begin precipitating. The reaction mixture was slowly cooled to room temperature and stirring was continued at about 5 °C for 75 minutes. The precipitated solid was collected by filtration, washed with ethyl acetate (4 mL) and subsequently dried at room temperature and under reduced pressure. The resulting product was suspended in 2-propanol (5 mL) and stirred at 50 °C for 90 minutes, then cooled to room temperature and continued stirring for 1 hour, and finally at 0-5 °C for another 1 hour. The solid formed was collected by filtration, washed with cold 2-propanol (5 mL) and dried at room temperature and under reduced pressure to afford 4-methyl-1-(3-nitro-5-(trifluoromethyl)phenyl)-1H-imidazole methanesulfonate as a beige solid. Yield: 54.1% (HPLC purity: 99.5 area%), melting point: 208-213 °C.
Yield:917391-29-8 54.1%
Reaction Conditions:
in ethyl acetate at 5 - 50; for 1.25 h;
Steps:
12
Example 12 4-Methyl-1 -(3-nitro-5-trif luoromethyl-phenyl)-1 H-imidazole methanesulfonic acid salt (XXIX)Crude 4-methyl-1-(3-nitro-5-trifluoromethyl-phenyl)-1 H-imidazole (IX) (1.85 g, 6 mmol, 88 area% purity in HPLC) is dissolved in ethyl acetate (6 ml_) at about 50 0C. To the stirred resulting black solution is slowly added methanesulfonic acid (0.397 ml_, 6 mmol) at about 500C. At the end of the addition a bright solid starts to precipitate. The mixture is allowed to slowly cool down to room temperature and is further stirred at about 5 0C for 75 minutes. The solid formed is filtered, washed with ethyl acetate (4 ml_) and dried at room temperature and reduced pressure. A suspension of the obtained material in 2-propanol (5 mL) is stirred at 50 0C for 90 minutes, is allowed to cool down to room temperature, stirred for 1 hour and at 0-50C for another hour. The solid formed is filtered, washed with cold 2-propanol (5 mL) and dried at room temperature and reduced pressure to afford 4-methyl-1-(3-nitro-5- trifluoromethyl-phenyl)-1H-imidazole methanesulfonic acid salt as a beige solid. -Yield: 54.1% (HPLC purity: 99.5 area%), Melting point: 208-213°C
References:
WO2006/135640,2006,A2 Location in patent:Page/Page column 24