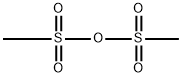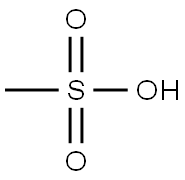
Methanesulfonic acid synthesis
- Product Name:Methanesulfonic acid
- CAS Number:75-75-2
- Molecular formula:CH4O3S
- Molecular Weight:96.11
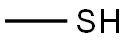
74-93-1
143 suppliers
$454.02/5MG

75-75-2
651 suppliers
$10.00/5g
Yield:75-75-2 100%
Reaction Conditions:
with dihydrogen peroxide;methanesulfonic acid in water at 40 - 110; for 10 h;
Steps:
1
Methyl mercaptan gas thus generated in the first part of the reaction was passed from the outlet tube simultaneously to a 4-necked 25L round bottomed glass vessel (similar set up as described in the first part of the reaction) through a dip tube containing 8. 5L of hydrogen peroxide (14%) (35mol) and 0. 125Kg of methanesulfonic acid as catalyst. While the stirrer was put on continuously, temperature of this reaction was also maintained at 40C (+ OR-5C) by manouevering heating and cooling continuously through out the reaction. Additional quantity OF 4L of hydrogen peroxide (30%) (35 mol) was added continuously after one hour of the mercaptan gas generation for over a period of 3.0 hrs. The reaction was maintained at 40C for another 6.0 hrs. At the end of the reaction this flask was heated to 100 to 110C to decompose any hydrogen peroxide that is not reacted completely. Distillation is carried out under reduced pressure and the METHANESULFONIC acid was obtained in quantitative yields (1.93Kg) accounting to the total amount of mercaptan charged into the second reactor from the first reactor.
References:
WO2004/58693,2004,A1 Location in patent:Page 5-6
34557-54-5
0 suppliers
inquiry

75-75-2
651 suppliers
$10.00/5g
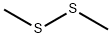
624-92-0
351 suppliers
$26.39/5ml

75-75-2
651 suppliers
$10.00/5g
2386-57-4
233 suppliers
$13.00/25g

75-75-2
651 suppliers
$10.00/5g