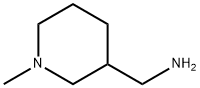
3-(Aminomethyl)-1-methylpiperidine synthesis
- Product Name:3-(Aminomethyl)-1-methylpiperidine
- CAS Number:14613-37-7
- Molecular formula:C7H16N2
- Molecular Weight:128.22
![Imidodicarbonic acid, 2-[(1-methyl-3-piperidinyl)methyl]-, 1,3-bis(1,1-dimethylethyl) ester](/CAS/20240320/GIF/672324-98-0.gif)
672324-98-0

14613-37-7
3-(Di-tert-butoxymethylamino)-1-methylpiperidine (500 mg, 1.52 mmol) was used as raw material and dissolved in a 1,4-dioxane solution (19.75 mL) of methanol (7.5 mL) and 10% (v/v) concentrated sulfuric acid. The reaction mixture was stirred at 25°C for 0.5 hours. Methanol (300 mL) was then added and BioRad AG1-X8 resin (OH type) was added until the pH reached 10. The resin was removed by filtration and the resin was washed with methanol (2 x 200 mL). The eluates were combined and evaporated to dryness, and the residue was purified by silica gel column chromatography (30 × 2.5 cm) to afford 3-(aminomethyl)-1-methylpiperidine (69.2 mg, 35%) using 10% concentrated ammonium hydroxide in methanol solution-dichloromethane as eluent. The product characterization data were as follows: FABMS: m/z 129.1 (MH+); HRFABMS: m/z 129.1392 (MH+), calculated value C7H17N2: m/z 129.1392; 1H NMR (CDCl3) δ 0.90 (2H, m, CH2), 1.65 (2H, m, CH2), 1.72 (1H, m. CH), 1.79 (1H, m, CH2), 1.91 (1H, m, CH2), 2.30 (3H, s, NCH3), 2.64 (2H, m, CH2), 2.82 (1H, m, CH2NH2), 2.92 (1H, m, CH2NH2); 13C NMR (CDCl3) δ CH3: 46.7; CH2: 25.2, 28.0, 46.0, 36.4, 60.3; CH: 39.9.
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

14613-37-7
109 suppliers
$45.00/25mg
Yield: 35%
Reaction Conditions:
with sulfuric acid in 1,4-dioxane;methanol at 25; for 0.5 h;
Steps:
244.A A. 3-(AMINOMETHYL)-1-METHYLPIPERIDINE
3- (DI-TERT-BUTOXYCARBONYLAMINO)-1-METHYLPIPERIDINE (500mg, 1. 52mmoles) (from Preparative Example 244, Step B above) was dissolved in methanol (7.5mL) and 10% (v/v) CONC. sulfuric acid in 1,4-dioxane (19. 75mL) was added. The solution was stirred at 25 C for 0. 5H. Methanol (300mL) was added, followed by BioRad AG1-X8 resin (OH FORM) until the pH was-10. The resin was filtered off and washed with METHANOL (2X200ML). The combined eluates were evaporated to dryness and the residue was chromatographed on a silica gel column (30x2.5cm) using 10% (10% CONC. ammonium hydroxide in METHANOL)-DICHLOROMETHANE as the eluant to give 3-(aminomethyl)-1- methylpiperidine (69.2mg, 35%): FABMS: m/z 129.1 (MH+) ; HRFABMS: m/z 129.1392 (MH+). CALCD. FOR C7H17N2 : m/z 129. 1392 ; 8H (CDC13) 0.90 (2H, m, CH2), 1.65 (2H, m, CH2), 1.72 (1 H, M, CH), 1.79 (1H, m, CH2), 1. 91 (1H, m, CH2), 2.30 (3H, s,-NCH3), 2.64 (2H, m, CH2), 2.82 (1 H, M,-CH2NH2) and 2.92 ppm (1 H, M,-CH2NH2) ; 8c (CDCl3) CH3 : 46.7 ; CH2 : 25.2, 28.0, 46. 3, 56.4, 60.3 ; CH: 39.9.
References:
SCHERING CORPORATION;PHARMACOPEIA, INC. WO2004/22561, 2004, A1 Location in patent:Page 109
3731-52-0
345 suppliers
$5.00/5g

14613-37-7
109 suppliers
$45.00/25mg