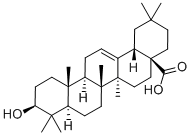
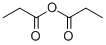
3-beta-Hydroxy-olean-12-en-28-oic acid propionate synthesis
- Product Name:3-beta-Hydroxy-olean-12-en-28-oic acid propionate
- CAS Number:107304-64-3
- Molecular formula:C33H52O4
- Molecular Weight:512.76
Yield:107304-64-3 87%
Reaction Conditions:
with pyridine;4-dimethylaminopyridine in dichloromethane at 20; for 12 h;
Steps:
4.2.1 General procedure for the syntheses of compounds 4-6
General procedure: OA/UA/GA (1 equiv, 1.10mmol) was dissolved in Pyridine: DCM (7mL:1 mL). Propionic anhydride (3 equiv, 0.43g, 3.30mmol) and DMAP (0.1 equiv, 0.013g, 0.11mmol) were then added and the resulting mixture was stirred at room temperature for 12h. The reaction mixture was evaporated under reduced pressure below 50°C and the resulting residue was extracted with EtOAc (100mL). The organic layer was washed sequentially with 2N HCl (50mL), H2O, saturated aqueous NaHCO3, and brine. The resulting mixture was dried over Na2SO4, filtered and concentrated. The residue was purified by silica gel column chromatography using petroleum ether/EtOAc (5/1) as eluent to give compound 4-6 3β-Propionyloxy-olean-12-en-28-oic acid (4). Yield: 0.49g, 87.00%, white solid. 1H NMR (500MHz, CDCl3): δ 0.73, 0.84, 0.85, 0.89, 0.91, 0.93, 1.12 (7s, each 3H, 7×CH3), 0.83-1.99 (m, 26H), 2.29-2.34 (m, 2H, CH2COO), 2.80 (dd, J=4.5, 13.8Hz, 1H, H-18), 4.47-4.51 (m, 1H, H-3), 5.26 (t, J=3.6Hz, 1H, H-12). 13C NMR (125MHz, CDCl3): δ 9.7, 15.7, 17.0, 17.5, 18.4, 23.1, 23.7, 23.8, 23.9, 26.2, 27.9, 28.3, 28.4, 30.9, 32.7, 32.8, 33.3, 34.0, 37.3, 38.0, 38.3, 39.5, 41.1, 41.8, 46.1, 46.8, 47.8, 55.5, 80.9, 122.8, 143.9, 174.6, 184.7. HRMS (ESI) m/z: calcd for C33H52O4Na [M+Na]+ 535.3763; found 535.3757
References:
Yin, Yudong;Sheng, Lixin;Zhang, Juzheng;Zhang, Liqiong;Liu, Jingjing;Wen, Xiaoan;Liu, Yanghan;Si, Yang;Cheng, Keguang [Bioorganic Chemistry,2022,vol. 126,art. no. 105865]