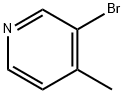
3-Bromo-4-methylpyridine synthesis
- Product Name:3-Bromo-4-methylpyridine
- CAS Number:3430-22-6
- Molecular formula:C6H6BrN
- Molecular Weight:172.02
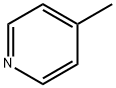
108-89-4

3430-22-6
The general procedure for the synthesis of 4-methyl-3-bromopyridine from 4-methylpyridine was as follows: 0.054 mol of 4-methylpyridine was added to a 60 mL constant pressure dropping funnel. Under nitrogen protection and stirring conditions at room temperature, it was slowly added dropwise to a mixture consisting of 0.07 mol AlCl3 and 0.01 mol potassium bromide, and the reaction mixture was stirred for 1 hour. A condensing unit was installed, heated to 120°C, and 0.07 mol of bromine was added slowly dropwise for about 1 hour dropwise. Heating and stirring were continued at 120°C for 26 hours. Upon completion of the reaction, the reaction solution was cooled to room temperature and slowly poured into crushed ice with stirring. The pH was adjusted to neutral by the addition of sodium hydroxide solution and stirred until completely dissolved. The aqueous layer was extracted with dichloromethane, the organic layers were combined and the solvent was recovered by steam distillation. The resulting oily product was purified by column chromatography (eluent ratio of VP petroleum ether: ethyl acetate = 6:1) to give 5.3 g of 3-bromo-4-methylpyridine in the form of a tan oil with a purity of 99.9% and a yield of 57%.
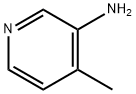
3430-27-1
459 suppliers
$13.43/1gm:

3430-22-6
418 suppliers
$6.00/1g
Yield:3430-22-6 95%
Reaction Conditions:
Stage #1: 3-amino-4-methylpyridinewith hydrogen bromide;bromine at -5;
Stage #2: with sodium nitrite in water at 0;
Stage #3: with sodium hydroxide in water at 0 - 20; pH=9; for 0.5 h;
Steps:
1.2; 2.2; 3.2 Preparation of 4-methyl-3-bromopyridine
2-Methyl-4-aminopyridine (10.8 g, 0.1 mol) was added to 48% HBr (46 ml, 0.4 mol) under cooling in an ice salt bath. After the addition was completed, it was cooled to -5 ° C and slowly added dropwise. Bromine (15ml, 0.3mol), 30-35min addition, then add 40g of 40% sodium nitrite solution below 0 ° C, add in 1-1.1h, continue to stir at 0 ° C for 30min, then Add 50% sodium hydroxide solution slowly below 20 °C to adjust the pH of the solution to 9, the reaction solution is extracted with ethyl acetate, the substrate is dried over anhydrous sodium sulfate, filtered with suction and concentrated to give 4-methyl-3-bromopyridine, the molar yield was 95%.
References:
CN109761891,2019,A Location in patent:Paragraph 0014; 0017; 0019; 0020; 0022; 0023; 0025

108-89-4
344 suppliers
$14.00/25mL

3430-22-6
418 suppliers
$6.00/1g

90731-85-4
0 suppliers
inquiry

3430-22-6
418 suppliers
$6.00/1g
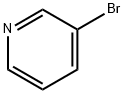
626-55-1
574 suppliers
$5.00/5/ G

3430-22-6
418 suppliers
$6.00/1g
