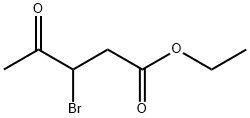
3-BROMO-4-OXO-PENTANOIC ACID ETHYL ESTER synthesis
- Product Name:3-BROMO-4-OXO-PENTANOIC ACID ETHYL ESTER
- CAS Number:54260-84-3
- Molecular formula:C7H11BrO3
- Molecular Weight:223.06

539-88-8
374 suppliers
$5.00/5g

54260-84-3
14 suppliers
inquiry
Yield:54260-84-3 43%
Reaction Conditions:
with bromine in diethyl ether at 0; for 3 h;
Steps:
7.A Step A: ethyl 3-bromo-4-oxopentanoate
To a solution of ethyl 4-oxopentanoate (4.92 mL, 34.7 mmol) in Et2O (180 mL) at about 0° C. was added bromine (1.8 mL, 34.7 mmol) in Et2O (10 mL) and the reaction was stirred for about 3 h. To the reaction mixture was added saturated aqueous sodium thiosulfate (50 mL) and stirred for about 10 min. The reaction was warmed to ambient temperature. The organic layer was separated and the aqueous layer was back extracted with Et2O (30 mL). The combined organic layers were washed with water (20 mL), dried over MgSO4, filtered and concentrated under reduced pressure. The resulting oil was purified by column chromatography (SiO2, EtOAc/heptane 100:0 to 0:20) to afford ethyl 3-bromo-4-oxopentanoate (3.29 g, 14.6 mmol, 43%). 1H NMR (CDCl3) δ 4.67-4.33 (m, 1H), 4.15 (q, J=7.1 Hz, 2H), 3.29-3.22 (m, 1H), 2.90-2.86 (m, 1H), 2.42 (s, 3H), 1.26 (t, J=7.1 Hz, 3H).
References:
US2014/179676,2014,A1 Location in patent:Paragraph 0961
123-76-2
497 suppliers
$5.00/25g

54260-84-3
14 suppliers
inquiry