
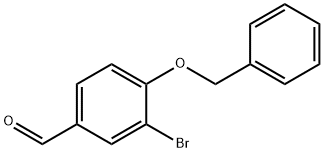
3-Bromo-4-(phenylmethoxy)-benzonitrile synthesis
- Product Name:3-Bromo-4-(phenylmethoxy)-benzonitrile
- CAS Number:317358-76-2
- Molecular formula:C14H10BrNO
- Molecular Weight:288.14
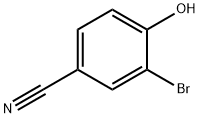
2315-86-8
191 suppliers
$7.00/250mg

100-39-0
429 suppliers
$10.00/10g

317358-76-2
9 suppliers
inquiry
Yield:317358-76-2 95%
Reaction Conditions:
with potassium carbonate in acetone; for 20 h;Inert atmosphere;Reflux;
Steps:
Synthesis of 4-(Benzyloxy)-3-bromobenzonitrile (8)
To a solution of 4-hydroxy-3-bromobenzonitrile (2.43g, 12.3mmol, 1.0 equiv) in acetone (25mL) was added potassium carbonate (5.09g, 36.8mmol, 3.0 equiv) and benzyl bromide (2.31g, 1.6mL, 13.5mmol, 1.07 equiv) and the mixture was stirred at reflux for 20h. After cooling to room temperature, the solvent was filtered and evaporated and redissolved in EtOAc (10mL) followed by washing with water (2×10mL). The combined organic layer was dried (MgSO4) and concentrated in vacuo. The crude product was purified by recrystallization from CH2Cl2 to afford the title compound 8 as a pale yellow solid (3.34g, 95%). The spectroscopic data of this compound matched with those in the literature [18]. Rf=0.27 (EtOAc:PS=15:85). mp: 98-100°C. IR (neat): νmax 3062, 2224, 1595, 1492, 1452, 1381, 1258, 998, 812, 737, 695cm-1. 1H NMR (500MHz, CDCl3): δ 7.84 (d, J=2.1Hz, 1H, H-2), 7.54 (dd, J=8.6, 2.1Hz, 1H, H-6), 7.47-7.32 (m, 5H, ArH), 6.97 (d, J=8.6Hz, 1H, H-5), 5.22 (s, 2H, PhCH2O). 13C NMR (125MHz, CDCl3): δ 158.6 (C-4), 136.9 (C-2), 135.3 (ArC), 133.1 (C-6), 128.9 (2× ArCH), 128.5 ArCH), 127.1 (2× ArCH), 117.8 (C≡N), 113.5 (C-5), 113.0 (C-3), 105.6 (C-1), 71.1 (PhCH2O).
References:
Thaima, Thanaphat;Willis, Anthony C.;Pyne, Stephen G. [Tetrahedron,2019,vol. 75,# 36,art. no. 130476]

2315-86-8
191 suppliers
$7.00/250mg

100-44-7
659 suppliers
$13.50/250G

317358-76-2
9 suppliers
inquiry
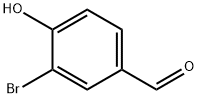
69455-12-5
49 suppliers
$31.00/250mg

317358-76-2
9 suppliers
inquiry
2973-78-6
224 suppliers
$14.00/5g

317358-76-2
9 suppliers
inquiry

100-39-0
429 suppliers
$10.00/10g

317358-76-2
9 suppliers
inquiry