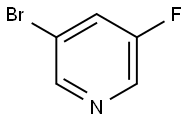
3-bromo-5-(tetrahydro-2H-pyran-4-yloxy)pyridine synthesis
- Product Name:3-bromo-5-(tetrahydro-2H-pyran-4-yloxy)pyridine
- CAS Number:422557-23-1
- Molecular formula:C10H12BrNO2
- Molecular Weight:258.11
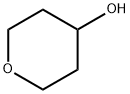
2081-44-9
301 suppliers
$6.00/1g
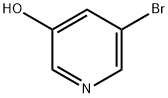
74115-13-2
410 suppliers
$10.00/1g

422557-23-1
10 suppliers
inquiry
Yield:422557-23-1 87%
Reaction Conditions:
Stage #1: Tetrahydro-pyran-4-ol;5-bromopyridine-3-olwith triphenylphosphine;diethylazodicarboxylate in toluene at 60 - 115; for 19 h;
Stage #2: with hydrogenchloride in water;
Stage #3: with sodium hydroxide in water at 5; pH=13;
Steps:
9
Example 9: Synthesis of 3-bromo-5-(tetrahydro-2H-pyran-4-yloxy)pyridine (12); A solution of 5-bromopyridin-3-ol (146 g, 834 mmol), tetrahydro-2H-pyran-4-ol (128 g,1250 mmol), and triphenylphosphine (329 g, 1250 mmol) in toluene (2.0 L) was heated to reflux, and 750 mL of distillate was removed via a Dean-Stark trap. The reaction mixture was cooled to 60 0C, and 547 g (1.25 mol) of a 40% (w/w) solution of DEAD in toluene was added drop-wise over a 1 hour period. The addition was exothermic with the reactor temperature at the end of addition near 95 0C. The reaction mixture was stirred at 115 0C for 18 h, and a portion of the reaction solution was sampled and analyzed by HPLC to establish that the reaction was complete. Upon completion of reaction, 500 mL of solvent was removed by distillation, and the pot residue was cooled to ambient temperature. This organic layer was washed with 10% aqueous sodium hydroxide (2 x 0.50 L) and concentrated under vacuum to produce a viscous oil, which was dissolved in 2N hydrochloric acid (1.0 L). Diatomaceous earth (100 g) was added with stirring and the resulting suspension was filtered. The pad was rinsed with 2N hydrochloric acid (1.0 L), and the filtrates were combined and extracted with diisopropyl ether (500 mL). The diisopropyl ether layer was discarded, and the aqueous layer was treated with carbon black (10 g) and stirred at 45-50 0C for 1 h. The suspension was filtered through a pad of diatomaceous earth (25 g). The filtrate was collected, cooled to 5 0C, and the pH adjusted with 50% aqueous sodium hydroxide (250 mL) to pH=13. The solution was extracted twice with chloroform (1.0 L, 600 mL), and the chloroform extracts were combined and concentrated under vacuum to give 12 as a dark red viscous oil/low melting solid (187 g, 87%), which was used without further purification. 1H NMR (CDCI3, 400 MHz) δ 8.29 (s, 1 H), 8.24 (s, 1 H), 7.38 (s, 1 H), 4.52 (m, 1 H), 3.98 (m, 2H), 3.60 (m, 2H), 2.05 (m, 2H), 1.81 (m, 2H).
References:
WO2010/65447,2010,A2 Location in patent:Page/Page column 38

2081-44-9
301 suppliers
$6.00/1g

74115-13-2
410 suppliers
$10.00/1g
108-20-3
592 suppliers
$25.00/25mL
1972-28-7
398 suppliers
$15.00/5g

422557-23-1
10 suppliers
inquiry