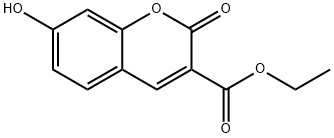
3-CARBETHOXYUMBELIFERONE synthesis
- Product Name:3-CARBETHOXYUMBELIFERONE
- CAS Number:6093-71-6
- Molecular formula:C12H10O5
- Molecular Weight:234.2

105-53-3
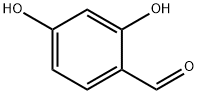
95-01-2

6093-71-6
The general procedure for the synthesis of ethyl 7-hydroxy-2-oxo-2H-benzopyran-3-carboxylate from 2,4-dihydroxybenzaldehyde (3.70 mmol) and diethyl malonate (7.90 mmol) was as follows: piperidine (10 drops) was added to a solution of diethyl malonate of 2,4-dihydroxybenzaldehyde and the resulting mixture was stirred and reacted for 2 hours at room temperature. Upon completion of the reaction, the reaction mixture was acidified with 10% aqueous hydrochloric acid solution (5 mL). The precipitated solid was collected by filtration and washed with cold water (10 mL). The crude product was purified by fast column chromatography using dichloromethane:ethyl acetate (70:30, v/v) as eluent to afford the target compound ethyl 7-hydroxy-2-oxo-2H-chromene-3-carboxylate (0.735 g, 97% yield). The structure of the product was confirmed by 1H NMR (400 MHz, DMSO-d6) and 13C NMR (100 MHz, DMSO-d6): 1H NMR δ 1.28 (t, J = 7.1 Hz, 3H), 4.24 (q, J = 7.1 Hz, 2H), 6.71 (d, J = 1.8 Hz, 1H), 6.83 (dd, J = 1.8, 8.3 Hz, 1H), 7.74 (d, J = 8.3 Hz, 1H), 8.66 (s, 1H), 11.07 (s, 1H); 13C NMR δ 14.1, 60.8, 101.7, 110.4, 113.9, 120.0, 132.1, 149.4, 156.3, 157.0, 162.9, 164.0.
Yield: 97%
Reaction Conditions:
with piperidine at 20; for 2 h;Knoevenagel condensation;
Steps:
Ethyl 7-hydroxy-2-oxo-2H-chromene-3-carboxylate (3)6
To a solution of 2,4-dihydroxybenzaldehyde (3.70 mmol) in diethyl malonate (7.90 mmol) piperidine (10 drops) was added, and the resulting solution was stirred for 2 hours at r.t. The resulting solution was then acidified with an aqueous solution of HCl10% (5 mL). The precipitate was filtrated and washed with cold water (10 mL). The desired product was purified by flash chromatography using dichloromethane : ethyl acetate (70: 30) as eluent furnishing 3 (0.735g) in 97% yield. 1H NMR (400 MHz, DMSO-d6) : 1.28 (t, J=7.1 Hz, 3H), 4.24 (q, J=7.1 Hz, 2H), 6.71 (d, J= 1.8Hz, 1H), 6.83 (dd, J= 1.8, 8.3Hz, 1H), 7.74 (d, J= 8.3Hz, 1H), 8.66 (s, 1H), 11.07 (s, OH). 13C NMR (100 MHz, DMSO-d6): 14.1, 60.8, 101.7, 110.4, 112.0, 113.9, 132.1, 149.4, 156.3, 157.0, 162.9, 164.0.
References:
Vieira, Lucas C.C.;Paixão, Márcio Weber;Corrêa, Arlene G. [Tetrahedron Letters,2012,vol. 53,# 22,p. 2715 - 2718] Location in patent:experimental part
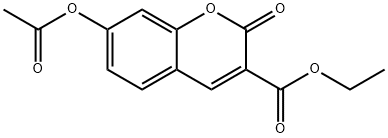
13209-77-3
14 suppliers
inquiry

6093-71-6
109 suppliers
$25.00/5mg
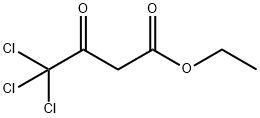
3702-98-5
58 suppliers
$17.67/250mgs:

95-01-2
493 suppliers
$10.00/1g

6093-71-6
109 suppliers
$25.00/5mg

105-53-3
793 suppliers
$5.00/25g

95-01-2
493 suppliers
$10.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

6093-71-6
109 suppliers
$25.00/5mg

105-56-6
461 suppliers
$10.00/5ml

95-01-2
493 suppliers
$10.00/1g

6093-71-6
109 suppliers
$25.00/5mg