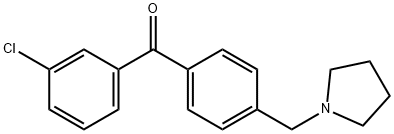
3-CHLORO-4'-PYRROLIDINOMETHYL BENZOPHENONE synthesis
- Product Name:3-CHLORO-4'-PYRROLIDINOMETHYL BENZOPHENONE
- CAS Number:898776-32-4
- Molecular formula:C18H18ClNO
- Molecular Weight:299.79
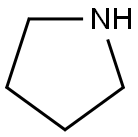
123-75-1
574 suppliers
$10.00/5g
-](/CAS/20210305/GIF/945657-22-7.gif)
945657-22-7
0 suppliers
inquiry

898776-32-4
10 suppliers
$433.00/1g
Yield:898776-32-4 73%
Reaction Conditions:
with triethylamine in acetonitrile at 0; for 1 h;
Steps:
1 (3-Chlorophenyl)[4-(pyrrolidin-1-ylmethyl)phenyl]methanone (5a)
To a stirred solution of 4a (3.5 g, 11.30 mmol) in dry acetonitrile (75 mL) was added pyrrolidine (1.41 mL, 16.96 mmol) and triethylamine (3.14 mL, 22.61 mmol) at 0° C. and the resulting mixture was allowed to stir for 1 h at 0° C. Thereafter, the reaction was quenched with 2 mL of water and the solvent was evaporated under reduced pressure. The residue was treated with water and extracted with chloroform (2*40 mL). The combined organic layers were washed with brine, dried over sodium sulphate and evaporated. The residue was chromatographed (2% methanol in dichloromethane) to afford 5a as a yellow viscous oil (2.50 g, 73%); 1H NMR (200 MHz, CDCl3) δ 7.70-7.56 (m, 3H), 7.49-7.13 (m, 5H), 3.62 (s, 2H), 2.47 (m, 4H), 1.73 (m, 4H); ESI MS m/z 300 (M+H)+.
References:
US2010/93726,2010,A1 Location in patent:Page/Page column 9-10
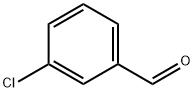
587-04-2
433 suppliers
$7.00/25g

898776-32-4
10 suppliers
$433.00/1g
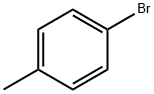
106-38-7
410 suppliers
$14.00/5g

898776-32-4
10 suppliers
$433.00/1g
13395-60-3
16 suppliers
$294.00/1g

898776-32-4
10 suppliers
$433.00/1g