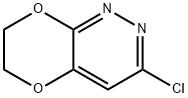
3-CHLORO-6,7-DIHYDRO[1,4]DIOXINO[2,3-C]PYRIDAZINE synthesis
- Product Name:3-CHLORO-6,7-DIHYDRO[1,4]DIOXINO[2,3-C]PYRIDAZINE
- CAS Number:943026-40-2
- Molecular formula:C6H5ClN2O2
- Molecular Weight:172.57
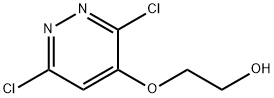
17284-80-9
![3-CHLORO-6,7-DIHYDRO[1,4]DIOXINO[2,3-C]PYRIDAZINE](/CAS/GIF/943026-40-2.gif)
943026-40-2
General procedure for the synthesis of 3-chloro-6,7-dihydro[1,4]dioxohexa[2,3-c]pyridazine from the compound (CAS:17284-80-9): (c) 2-[(3,6-dichloro-4-pyridazinyl)oxy]ethanol (15.46 g; 0.0703 mol) containing the bromine derivative was dissolved in anhydrous dioxane (1.2 L). Lithium hydride (2.3 g; 0.28 mol) was added in batches and stirred under argon protection at room temperature for 1 h. The reaction was then heated to 110 °C overnight. Upon completion of the reaction, the reaction was quenched with wet dioxane and then quenched with ice water. The reaction solution was concentrated to half of the original volume, the pH was adjusted to 8 with 5 M hydrochloric acid and then evaporated to dryness. After addition of water, the residue was extracted with chloroform and the organic phase was dried over sodium sulfate and concentrated to give a white solid (12.4 g, ~77% yield, containing ~15% bromine derivative). Mass spectrometric analysis (positive ion electrospray) showed m/z 173/5 (Cl MH+) and 217/9 (Br MH+).

17284-80-9
1 suppliers
inquiry
![3-CHLORO-6,7-DIHYDRO[1,4]DIOXINO[2,3-C]PYRIDAZINE](/CAS/GIF/943026-40-2.gif)
943026-40-2
24 suppliers
inquiry
Yield:943026-40-2 77%
Reaction Conditions:
with lithium hydride in 1,4-dioxane at 20; for 1 h;
Steps:
10.A.c
(c) 3-Chloro-6,7-dihydro[1,4]dioxino[2,3-c]pyridazineA solution of 2-[(3,6-dichloro-4-pyridazinyl)oxy]ethanol containing some bromo-derivative (15.46 g; 0.0703 mol) in dry dioxan (1.2 L) was treated with lithium hydride (2.3 g; 0.28 mol) in portions and stirred at room temperature for 1 hour under argon, then heated at 110° C. overnight. The reaction mixture was quenched with wet dioxan, then iced-water. The solution was evaporated to half volume, taken to pH 8 with 5M hydrochloric acid and evaporated to dryness. Water was added and the residue was extracted 5× with chloroform, dried (sodium sulphate) and evaporated to afford a white solid (12.4 g, ca. 77%) (containing ca. 15% of a bromo species).MS (+ve ion electrospray) m/z 173/5 (Cl MH+); 217/9 (Br MH+)
References:
US2008/221110,2008,A1 Location in patent:Page/Page column 18