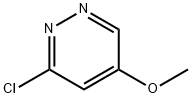
3-CHLORO-5-METHOXYPYRIDAZINE synthesis
- Product Name:3-CHLORO-5-METHOXYPYRIDAZINE
- CAS Number:123696-02-6
- Molecular formula:C5H5ClN2O
- Molecular Weight:144.56
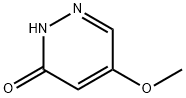
123696-01-5

123696-02-6
5-Methoxypyridazin-3(2H)-one (250 mg, 1.98 mmol) was used as starting material and suspended in phosphoryl chloride (1.5 mL, 16 mmol). The reaction mixture was stirred at 100 °C for 10 min. After completion of the reaction, the mixture was slowly poured into ice water and the pH was adjusted to alkaline by adding sodium carbonate. Subsequently, the mixture was extracted with ether and the organic phases were combined and dried with anhydrous magnesium sulfate. After filtration, the solvent was removed by distillation under reduced pressure. The resulting residue was recrystallized with 1,2-dichloroethane to give 130 mg of 3-chloro-5-methoxypyridazine in 45% yield. The product was analyzed by LC-MS (Method 5): r = 0.85 mm; MS (ESIpos): m/z = 145 [M + H]+. 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 2.495 (0.78), 2.499 (1.07), 2.503 (0.84), 3.317 (11.94). 3.944 (16.00), 7.528 (2.28), 7.534 (2.29), 8.988 (2.34), 8.994 (2.34).

124-41-4
731 suppliers
$12.00/25g
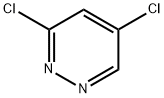
1837-55-4
183 suppliers
$23.00/250mg

123696-02-6
133 suppliers
$17.00/250mg
Yield:-
Reaction Conditions:
in methanol at 90; for 1 h;
Steps:
131.1
Example 131 2-Chloro-N-(1 -hydroxy-cyclohexylmethyl)-5-(5-methoxy-pyridazin-3- yloxy)-benzamide131.1 3-chloro-5-methoxyDyridazineTo a solution of 3,5-dichloropyridazine (300 mg) in MeOH (2 mL) was added a 5.4 M solution of sodium methoxide in MeOH (0.410 mL) and the reaction mixture was stirred for 1 h at 90°C. It was quenched with H20 and extracted with EtOAc. The organic phase was washed with a 5% solution of KHS04, a sat. solution of NaHC03 and brine, dried over MgS04 and concentrated in vacuo to give the crude titled compound as an orange solid.1H NMR ((CD3)2SO) δ: 9.01 (d, J = 2.4 Hz, 1 H), 7.55 (d, J = 2.4 Hz, 1 H), 3.96 (s, 3 H)
References:
ACTELION PHARMACEUTICALS LTD;HILPERT, Kurt;HUBLER, Francis;MURPHY, Mark;RENNEBERG, Dorte WO2012/114268, 2012, A1 Location in patent:Page/Page column 119

123696-01-5
90 suppliers
$60.00/100mg

123696-02-6
133 suppliers
$17.00/250mg
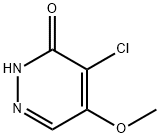
63910-43-0
110 suppliers
$38.00/1g

123696-02-6
133 suppliers
$17.00/250mg

67-56-1
790 suppliers
$7.29/5ml-f

124-41-4
731 suppliers
$12.00/25g

1837-55-4
183 suppliers
$23.00/250mg

123696-02-6
133 suppliers
$17.00/250mg
173206-12-7
7 suppliers
inquiry

123696-02-6
133 suppliers
$17.00/250mg