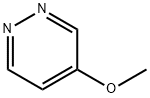
4-METHOXYPYRIDAZINE synthesis
- Product Name:4-METHOXYPYRIDAZINE
- CAS Number:20733-11-3
- Molecular formula:C5H6N2O
- Molecular Weight:110.11
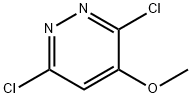
70952-62-4

20733-11-3
General procedure for the synthesis of 4-methoxypyridazine from 4-methoxy-3,6-dichloropyridazine: 10% palladium carbon (3 g) catalyst was added to a solution of methanol (500 mL) containing 3,6-dichloro-4-methoxypyridazine (30 g, 167.59 mmol, 1.00 equiv) and ammonium formate (31 g, 491.63 mmol, 2.93 equiv). The reaction mixture was stirred at room temperature and 1 atm overnight. Upon completion of the reaction, the catalyst was removed by filtration and the filtrate was concentrated under vacuum. The concentrated residue was ground in a solvent mixture of dichloromethane and methanol (500 mL, 10:1 v/v) and subsequently filtered to collect the solid. The filtrate was again concentrated under vacuum and the residue was purified by silica gel column chromatography with the eluent being a solvent mixture of ethyl acetate and petroleum ether (the ratio was tapered from 2:1 to 1:1), resulting in 15 g (81% yield) of 4-methoxypyridazine as a brown oil. Thin-layer chromatography (TLC) conditions: the ratio of petroleum ether to ethyl acetate was 2:1, and the Rf value was 0.1.

70952-62-4
88 suppliers
$18.00/250mg

20733-11-3
80 suppliers
$204.00/1g
Yield:20733-11-3 81%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen;ammonium formate in methanol at 20; under 760.051 Torr;
Steps:
86.1 Step 1 . 4-Methoxypyridazine.
To a solution of 3 ,6-dichloro-4- methoxypyridazine (30 g, 167.59 mmol, 1.00 equiv) and ammonium formate (3 1 g, 491.63 mmol, 2.93 equiv) in MeOH (500 mL) was added 10% palladium on carbon (3 g) catalyst. The mixture was stirred under 1 atmosphere of at rt overnight. The catalyst was removed by filtration and the filtrate was concentrated under vacuum. The residue was triturated in 500 mL of DCM MeOH ( 10: 1 ) and the solid material was filtered out. The filtrate was concentrated under vacuum and the residue was purified on a silica gel column eluted with ethyl acetate/petroleum ether (2:1 to 1: 1) to give 1 5 g (81%) of 4-methoxypyridazine as a brown oil. TLC: petroleum ether: ethyl acetate=2: 1 , Rf=0.1 .
References:
WO2013/127268,2013,A1 Location in patent:Page/Page column 99
123696-02-6
134 suppliers
$17.00/250mg

20733-11-3
80 suppliers
$204.00/1g
123696-01-5
90 suppliers
$60.00/100mg

20733-11-3
80 suppliers
$204.00/1g
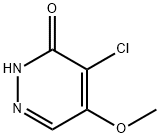
63910-43-0
110 suppliers
$38.00/1g

20733-11-3
80 suppliers
$204.00/1g

34361-96-1
0 suppliers
inquiry

20733-11-3
80 suppliers
$204.00/1g