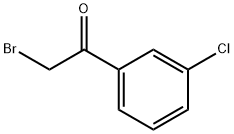
3-CHLOROPHENACYL BROMIDE synthesis
- Product Name:3-CHLOROPHENACYL BROMIDE
- CAS Number:41011-01-2
- Molecular formula:C8H6BrClO
- Molecular Weight:233.49
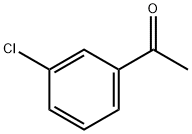
99-02-5

41011-01-2
Molecular sieve-dried 3-chloroacetophenone (154.6 g, 1 mol) was added to a four-necked flask with anhydrous methanol (310 mL) under nitrogen protection. Bromine (158.2 g, 0.99 mol) was slowly added dropwise under continuous stirring in the temperature range of 30 °C to 45 °C. The dropwise addition was controlled to be completed within 1 h. The reaction was carried out in a controlled manner. After the dropwise addition was completed, the reaction mixture was kept at the same temperature and stirring was continued for 10 minutes. Subsequently, water (160 g) was added and the reaction solution was cooled to -10°C to promote crystal precipitation. The precipitate was collected by filtration to give 250 g of crude product. The crude product was dissolved in heptane (750 g), washed twice sequentially with water (200 g) and the organic phase was dried with anhydrous magnesium sulfate. The desiccant was removed by filtration, and the filtrate was concentrated to give 212.5 g of 2-bromo-3-chloroacetophenone in 91.0% yield, and no impurities were detected.

99-02-5
291 suppliers
$9.00/1g

41011-01-2
148 suppliers
$6.00/250mg
Yield:41011-01-2 91%
Reaction Conditions:
with bromine in n-heptane;water
Steps:
5 EXAMPLE 5
EXAMPLE 5 Under a nitrogen atmosphere, 3'-chloroacetophenone (154.6 g, 1 mol) and anhydrous methanol (310 ml) dried with molecular sieves were charged in a four-necked flask. Bromine (158.2 g, 0.99 mol) was added dropwise with stirring at a temperature in a range from 30° to 45° C. over one hour, and the mixture was maintained at the same temperature for 10 minutes. After water (160 g) was added, the solution was cooled to -10° C. to precipitate crystals which were filtered to obtain 250 g of a crude cake. This crude cake was dissolved in heptane (750 g), and the solution was washed twice with water (200 g), dried over anhydrous magnesium sulfate, filtered, washed and then concentrated to obtain 212.5 g of 2-bromo-3'-chloroacetophenone. The yield was 91.0% and no impurity was detected.
References:
Sumika Fine Chemicals Company, Limited US5717116, 1998, A
2039-85-2
175 suppliers
$11.00/1g

41011-01-2
148 suppliers
$6.00/250mg
766-83-6
175 suppliers
$9.00/1g

41011-01-2
148 suppliers
$6.00/250mg
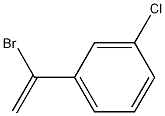
187409-14-9
1 suppliers
inquiry

41011-01-2
148 suppliers
$6.00/250mg
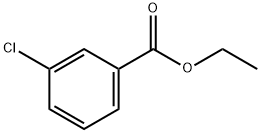
1128-76-3
125 suppliers
$8.00/1g

74-95-3
270 suppliers
$10.00/10g

41011-01-2
148 suppliers
$6.00/250mg