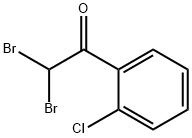
2-Bromo-2'-chloroacetophenone synthesis
- Product Name:2-Bromo-2'-chloroacetophenone
- CAS Number:5000-66-8
- Molecular formula:C8H6BrClO
- Molecular Weight:233.49
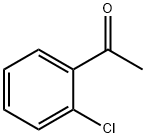
2142-68-9

5000-66-8
The general procedure for the synthesis of 2-bromo-2'-chloroacetophenone from o-chloroacetophenone is as follows: in a reaction system, 1.2 g (10 mmol) of o-chloroacetophenone 1a was mixed and stirred with 0.121 g (0.5 mmol) of Cu(NO3)2-3H2O while an oxygen bulb (ca. 0.5-1 L) was attached. Subsequently, an aqueous 8 mol/L hydrobromic acid solution (1.5 mL, 12 mmol) was slowly added dropwise to the mixture. The reaction mixture was stirred continuously at 70 °C and the reaction progress was monitored by TLC or GC. After completion of the reaction, the mixture was extracted with CH2Cl2. The organic extract was washed sequentially with 5% sodium sulfite solution, saturated sodium bicarbonate solution and water and finally dried with anhydrous magnesium sulfate. The solvent was removed under vacuum and the residue was purified by column chromatography (silica gel as stationary phase, petroleum ether/dichloromethane 3:1 v/v/v) to afford the target product 2-bromo-2'-chloroacetophenone (2a) in a yield of 1.81 g in 91%.

2142-68-9
402 suppliers
$6.00/10g

5000-66-8
275 suppliers
$14.00/1g
Yield:5000-66-8 99%
Reaction Conditions:
with bromine in water at 20; for 0.75 h;Large scale;
Steps:
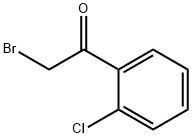
1.1 (1) Synthesis of α-bromo-2-chloroacetophenone (3)
Add 230.0g (1.5mol) o-chloroacetophenone and 1188mL water into a 3L three-necked flask, react at 20°C, add 84.4mL (1.6mol) liquid bromine dropwise within 0.5h, and stir for 15min. It was extracted with 400 mL of dichloromethane, and the organic layer was washed with 4.7 mol/L sodium carbonate aqueous solution and dried with anhydrous sodium sulfate. After filtering and evaporating the solvent under reduced pressure, 346.3 g of pale yellow oily liquid was obtained with a yield of 99%.
References:
CN112707829, 2021, A Location in patent:Paragraph 0036-0038
1056775-36-0
1 suppliers
inquiry

5000-66-8
275 suppliers
$14.00/1g
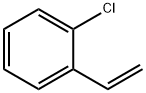
2039-87-4
162 suppliers
$7.00/1g

5000-66-8
275 suppliers
$14.00/1g
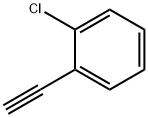
873-31-4
183 suppliers
$14.00/1g

5000-66-8
275 suppliers
$14.00/1g