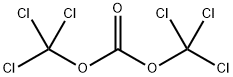
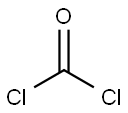
3-chlorophenyl chloroformate synthesis
- Product Name:3-chlorophenyl chloroformate
- CAS Number:49782-76-5
- Molecular formula:C7H4Cl2O2
- Molecular Weight:191.01
Yield:-
Reaction Conditions:
with triethylamine in 1,2-dichloro-ethane at 20;
Steps:
4.5. A general procedure for acylation of carbolines (method E)
General procedure: To a mixture of phosgene (or its analog, such as triphosgene, ~1equiv) and base (such as NEt3, DIEA, ~2-3 equiv.) in solvent (such asDCM, THF) at rt was added an alcohol (~1 equiv.). The mixture was stirred at rt for 1h to generate the chloroformate. To the mixture wasadded an amine 7 (~1-2 equiv, neat or a solution in DMF). The mixturestirred at rt for several hours before being quenched with methanol. Themixture was concentrated and purified by chromatography to providethe final compound.
References:
Baiazitov, Ramil Y.;Qi, Hongyan;Arasu, Tamil;Lennox, William;Cao, Liangxian;Weetall, Marla;Furia, Bansri;Zhuo, Jin;Choi, Soongyu;Kim, Min Jung;Sheedy, Josephine;Davis, Thomas;Moon, Young-Choon [European Journal of Medicinal Chemistry,2022,vol. 244,art. no. 114826]
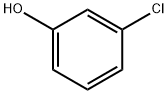
108-43-0
305 suppliers
$13.00/5g

49782-76-5
6 suppliers
inquiry