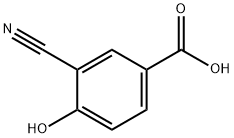
3-Cyano-4-hydroxybenzoic acid synthesis
- Product Name:3-Cyano-4-hydroxybenzoic acid
- CAS Number:70829-28-6
- Molecular formula:C8H5NO3
- Molecular Weight:163.13
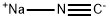
773837-37-9
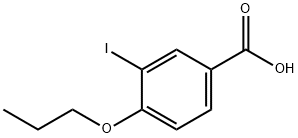
1131588-12-9

70829-28-6
General procedure for the synthesis of 3-carbonitrile-4-hydroxybenzoic acid from compound (CAS: 773837-37-9) and compound (CAS: 1131588-12-9): the product of Example 44 Step C (0.22 g, 0.72 mmol), NaCN (0.04 g, 0.8 mmol), and CuCN (0.07 g, 0.8 mmol) in anhydrous DMF (2 mL) was stirred at 110 °C for 2 h in a nitrogen atmosphere. Upon completion of the reaction, the mixture was evaporated to dryness under vacuum and the residue was suspended in water (10 mL) and the pH was adjusted with 1 M NaOH solution to about 10. The insoluble material was removed by filtration and the filtrate was acidified with dilute HCl to pH ~3. The product was extracted with dichloromethane (2 x 20 mL). The organic phases were combined and dried with anhydrous MgSO4, and the filtrate was filtered and evaporated to dryness under reduced pressure to give 3-carbonitrile-4-hydroxybenzoic acid (0.1 g, 67% yield) as a brown solid.1H-NMR (CDCl3) δ: 1.06-1.14 (m, 3H), 1.85-1.97 (m, 2H), 4.1-4.18 (m, 2H), 7.02 (d , 1H, J = 9 Hz), 8.24 (dd, 1H, J = 3, 9 Hz), 8.31 (d, 1H, J = 3 Hz).
Yield:70829-28-6 67%
Reaction Conditions:
Stage #1:sodium cyanide;3-iodo-4-propoxybenzoic acid with copper(I) cyanide in N,N-dimethyl-formamide at 110; for 2 h;Inert atmosphere;
Stage #2: with water;sodium hydroxide; pH=~ 10
Steps:
45.A
A mixture of the product of Example 44 Step C (0.22 g; 0.72 mmol), NaCN (0.04 g; 0.8 mmol) and CuCN (0.07 g; 0.8 mmol) in anhydrous DMF (2 ml) was stirred at ~ 1 1 O0C for 2 h under N2. This was evaporated to dryness in vacuo and the residue was suspended in H2O (10 ml) and pH was adjusted to ~ 10 with 1 M NaOH. The insoluble material was removed by filtration and the filtrate was acidified to pH ~ 3 with diluted HCI and the product was taken up by CH2CI2 (2 x 20 ml). The organic phase was dried over anhydrous MgSO4, filtered and the filtrate evaporated to dryness under reduced pressure to give the title compound (0.1 g; 67%) as brownish solid. 1H- NMR (CDCI3) 1.06 - 1 .14 (m, 3H); 1.85 - 1.97 (m, 2H); 4.1 - 4.18 (m, 2H); 7.02 (d, 1 H, J = 9 Hz); 8.24 (dd, 1 H, J = 3, 9 Hz); 8.31 (d, 1 H, J = 3 Hz).
References:
AKAAL PHARMA PTY LTD;GILL, Gurmit, S.;GROBELNY, Damian, W. WO2010/42998, 2010, A1 Location in patent:Page/Page column 109
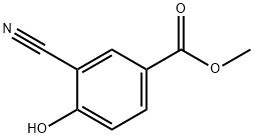
156001-68-2
89 suppliers
inquiry

70829-28-6
87 suppliers
inquiry
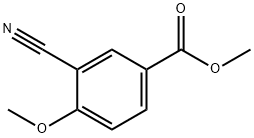
25978-74-9
97 suppliers
$43.00/1g

70829-28-6
87 suppliers
inquiry
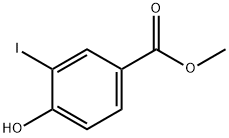
15126-06-4
140 suppliers
$6.00/250mg

70829-28-6
87 suppliers
inquiry
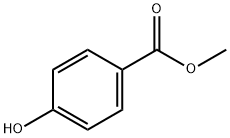
99-76-3
813 suppliers
$5.00/25g

70829-28-6
87 suppliers
inquiry