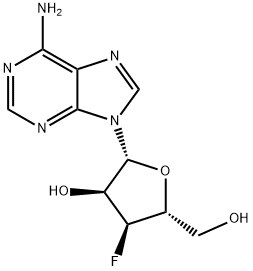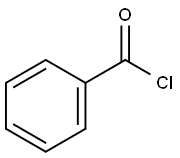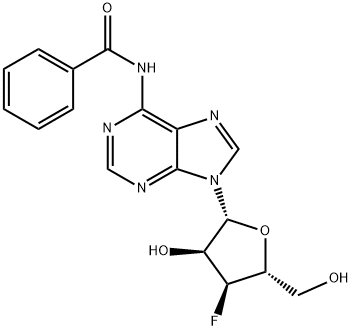
3'-Deoxy-3'-fluoro-N6-benzoyladenosine synthesis
- Product Name:3'-Deoxy-3'-fluoro-N6-benzoyladenosine
- CAS Number:129054-67-7
- Molecular formula:C17H16FN5O4
- Molecular Weight:373.34
Yield:129054-67-7 2.75 g
Reaction Conditions:
with pyridine;chloro-trimethyl-silane at 0 - 20; for 5 h;
Steps:
1.7 Step 7
Preparation of N-(9-((2R,3S,4S,5R)-4-fluoro-3-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-9H-purin-6-yl)benzamide (i14):
To compound 70 i13 (3.88 g, 14.41 mmol) in 65 pyridine (65 mL) at 0° C. was added 73 benzoyl chloride (8.36 mL, 72.1 mmol) slowly followed by TMSCl (9.21 mL, 72.1 mmol). The reaction mixture was stirred while warming to rt for 4 h. After another 1 h the solution was quenched with 14 water (35 mL), followed by 74 conc. NH4OH (17 mL) after 5 min resulting in a pale tan solid. The mixture was diluted with water (100 mL) and extracted with MeTHF (3×75 mL). The combined organic phases were dried (Na2SO4), the drying agent filtered off and the filtrate concentrated in vacuo to a tan semi-solid crude material, which was purified by chromatography on silica gel (gradient elution 0-20% 29 MeOH/DCM) to give the desired 75 compound i14 (2.75 g): 1H NMR (400 MHz, CDCl3) δ 8.78 (s, 1H), 8.09 (s, 1H), 8.08-8.01 (m, 2H), 7.66 (t, J=7.4 Hz, 1H), 7.57 (t, J=7.5 Hz, 2H), 6.13 (br s, 1H), 5.92 (d, J=7.9 Hz, 1H), 5.41-5.11 (m, 2H), 4.60 (d, J=28.4 Hz, 1H), 4.13-3.98 (m, 2H), 3.86 (d, J=13.0 Hz, 1H). 19F NMR (376.4 MHz, CDCl3) δ -199.36; LCMS (Method G) Rt 0.72 mins; m/z 374.2 (M+H)+.
References:
US2019/185511,2019,A1 Location in patent:Paragraph 0559; 0572-0573
123484-12-8
10 suppliers
inquiry

129054-67-7
20 suppliers
inquiry

1009818-08-9
0 suppliers
inquiry

129054-67-7
20 suppliers
inquiry

1101107-81-6
0 suppliers
inquiry

129054-67-7
20 suppliers
inquiry