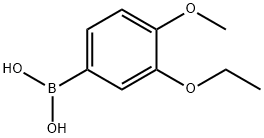
3-ETHOXY-4-METHOXYPHENYLBORONIC ACID synthesis
- Product Name:3-ETHOXY-4-METHOXYPHENYLBORONIC ACID
- CAS Number:915201-13-7
- Molecular formula:C9H13BO4
- Molecular Weight:196.01

5419-55-6
388 suppliers
$12.00/5g
52849-52-2
43 suppliers
$45.00/100mg

915201-13-7
38 suppliers
$71.00/250mg
Yield:915201-13-7 93%
Reaction Conditions:
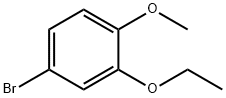
Stage #1: 1-Bromo-3-ethoxy-4-methoxybenzenewith tert.-butyl lithium in tetrahydrofuran;pentane at -78; for 1 h;
Stage #2: Triisopropyl borate in tetrahydrofuran;pentane at -78 - 20; for 16 h;
Stage #3: with hydrogenchloride;water in tetrahydrofuran;pentane; for 0.166667 h;
Steps:
5.117.1
5.117.1 3-Ethoxy-4-Methoxyphenylboronic Acid A mixture of 4-Bromo-2-ethoxy-1-methoxybenzene (4.00 g, 17.3 mmol) in THF (75 mL) was cooled to -78° C.; during cooling, a precipitate formed. t-BuLi (22.4 mL, 1.7 M in pentane, 38.1 mmol) was added dropwise, while maintaining the temperature at -78° C. The mixture was stirred at -78° C. for 1 hour following completion of the addition. B(Oi-Pr)3 (9.76 g, 51.9 mmol) was added. The mixture was allowed to gradually warm to room temperature, and then stirred under nitrogen for 16 hours. 3N HCl (20 mL) was added, and the mixture stirred for 10 minutes. The mixture was poured into water (100 mL) and extracted with diethyl ether (3×75 mL), and the combined ethereal layers were washed with water (3×75 mL), dried (MgSO4) and evaporated, providing 3.15 g of the product in 93% yield: 1H NMR (DMSO-d6) δ 1.32 (t, J=7.0 Hz, 3H), 3.75 (s, 3H), 3.99 (q, J=7.0 Hz, 2H), 6.90 (d, J=8.3 Hz, 1H), 7.35-7.37 (m, 2H).
References:
US2007/49618,2007,A1 Location in patent:Page/Page column 115

121-43-7
383 suppliers
$14.00/25mL

52849-52-2
43 suppliers
$45.00/100mg

915201-13-7
38 suppliers
$71.00/250mg
613-70-7
118 suppliers
$30.00/1g

915201-13-7
38 suppliers
$71.00/250mg
66037-04-5
18 suppliers
$60.00/100mg

915201-13-7
38 suppliers
$71.00/250mg
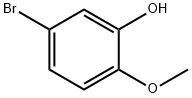
37942-01-1
244 suppliers
$6.00/5g

915201-13-7
38 suppliers
$71.00/250mg