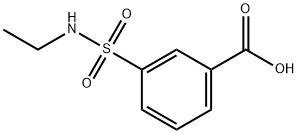
3-[(ETHYLAMINO)SULFONYL]BENZOIC ACID synthesis
- Product Name:3-[(ETHYLAMINO)SULFONYL]BENZOIC ACID
- CAS Number:7326-74-1
- Molecular formula:C9H11NO4S
- Molecular Weight:229.25
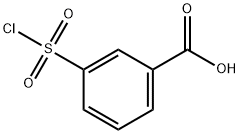
4025-64-3
206 suppliers
$6.00/1g
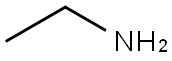
75-04-7
370 suppliers
$10.00/20mg
![3-[(ETHYLAMINO)SULFONYL]BENZOIC ACID](/CAS/GIF/7326-74-1.gif)
7326-74-1
25 suppliers
$45.00/100mg
Yield:-
Reaction Conditions:
in acetone;Reflux;
Steps:
2.1. Synthesis of Phenylacetic Acid and Trifluoromethylphenyl Substituted Benzamide Derivatives
General procedure: Dry benzoic acid (0.01 mol) was taken in round-bottom flask equipped with a stirrer in an ice bath chamber and cooled with maintaining the temperature between 10 to 15 °C. Chlorosulfonic acid (8.0 mL) was added and funnel was stoppered with calcium chloride filled with cotton wool tubes and carefully checked to ensure that there was no leaking.The mixture showed exothermic reaction and when whole benzoic acid was dissolved followed by the extinction of exothermic reaction, the flask was heated in a hot water bath at 70-80 °C for 2 h to complete the reaction. The mixture was cooled and transferred to 150 g crushed ice. 3-(chlorosulphonyl)benzoic acid was collected by vacuum filtration and washed with cold water and air dried. 3-(chlorosulphonyl)benzoic acid (0.01 mol) and commercially available aliphatic aromatic, and heteroaromatic amines(0.01 mol) were refluxed in acetone and the completion of reaction was monitored by TLC to get the respective sulphonamides of benzoic acid. The reaction mixture was cooled and precipitates obtained were washed and recrystallized. The different sulphonamides obtained (0.01 mol) were refluxed with thionyl chloride (0.01 mol) for 3 h. While the refluxing got over, the excess of thionyl chloride was distilled off to get the respective benzoyl chlorides. Phenylacetic acid derivatives (1-6) were obtained by refluxing the respective benzoyl chlorides (0.01 mol) and 2-aminophenylacetic acid (0.015 mol) in methanol. The products obtained after evaporation of acetone were recrystallized with ethanol. 2-trifluoromethylphenyl benzamide derivatives(7-26) were obtained by refluxing the respective benzoyl chlorides (0.01 mol) with 2-trifluoromethylaniline (0.015mol) in acetone. The product obtained after evaporation of acetone was recrystallized with ethanol.
References:
Grewal, Ajmer Singh;Lather, Viney;Pandita, Deepti;Bhayana, Garima [Letters in drug design and discovery,2017,vol. 14,# 11,p. 1239 - 1251]
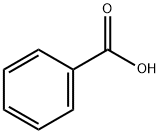
65-85-0
1321 suppliers
$10.00/25g
![3-[(ETHYLAMINO)SULFONYL]BENZOIC ACID](/CAS/GIF/7326-74-1.gif)
7326-74-1
25 suppliers
$45.00/100mg