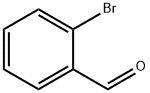
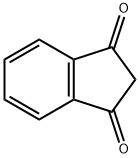
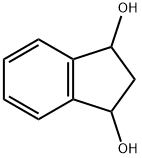
3-Hydroxy-1-indanone synthesis
- Product Name:3-Hydroxy-1-indanone
- CAS Number:26976-59-0
- Molecular formula:C9H8O2
- Molecular Weight:148.16
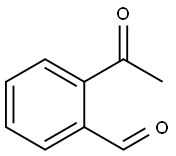
24257-93-0
51 suppliers
$75.00/500mg

26976-59-0
8 suppliers
inquiry
Yield:26976-59-0 94%
Reaction Conditions:
with dl-β-phenyl-α-alanine, potassium salt in dimethyl sulfoxide at 25; for 3 h;
Steps:
g. General procedure for the synthesis of substituted 3-hydroxyindanones (2)
General procedure: To a solution of 1 (1.00 mmol) in DMSO (3.00 mL), phenylalanine potassium salt (0.10 mmol) was added, and the mixture was stirred at room temperature (in case of substrate 1a-1r) or at 60 °C (in case of substrate 1s-1zb) till the completion of the reaction (monitored by TLC). After completion of the reaction, water (50 mL) was added followed by extraction with ethyl acetate (3 × 30 mL). The combined organic layer was separated, washed with water thrice then with brine, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The crude residue thus obtained was purified by column chromatography on silica gel using ethyl acetate/hexane as the eluting system.
References:
Chanda, Tanmoy;Chowdhury, Sushobhan;Anand, Namrata;Koley, Suvajit;Gupta, Ashutosh;Singh, Maya Shankar [Tetrahedron Letters,2015,vol. 56,# 8,p. 981 - 985] Location in patent:supporting information

30385-41-2
0 suppliers
inquiry

26976-59-0
8 suppliers
inquiry