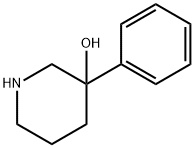
3-HYDROXY-3-PHENYL-PIPERIDINE synthesis
- Product Name:3-HYDROXY-3-PHENYL-PIPERIDINE
- CAS Number:23396-50-1
- Molecular formula:C11H15NO
- Molecular Weight:177.24
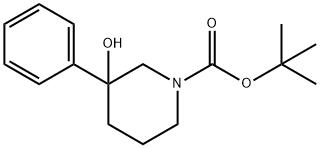
213923-81-0
31 suppliers
$95.00/100mg

23396-50-1
46 suppliers
$21.00/100mg
Yield: 97%
Reaction Conditions:
with toluene-4-sulfonic acid in dichloromethane at 20; for 2 h;
Steps:
1.d (d)
3-Phenylpiperidin-3-ol (4)
(d)
3-Phenylpiperidin-3-ol (4)
Compound 3 (469 mg, 1.7 mmol) and p-toluenesulfonic acid monohydrate (675 mg, 3.5 mmol) were dissolved in dichloromethane (8.5 mL) and stirred for 2 h at room temperature. 3 N Potassium hydroxide (10 mL) was added to the reaction mixture, and extracted with dichloromethane (10 mL, 4 times).
The combined organic layers were washed with saturated brine (50 mL), and the aqueous layer was back extracted with dichloromethane (30 mL, 4 times).
The combined organic layers were dried over sodium sulfate, filtered and concentrated under reduced pressure to afford Compound 4 (291 mg, 97%) as pale yellow solid.
1H NMR (300 MHz, CD3OD) δ 7.53-7.46 (2H, m), 7.37-7.29 (2H, m), 7.26-7.18 (1H, m), 3.08-2.99 (1H, m), 2.93-2.85 (1H, m), 2.81-2.72 (1H, m), 2.68-2.56 (1H, m), 2.13-1.80 (3H, m), 1.61-1.51 (1H, m); 13C NMR (75.5 MHz, CD3OD) δ 148.7, 129.2, 127.9, 125.8, 71.8, 57.4, 46.3, 37.2, 23.4; ESI TOF-MS m/z Calcd for C11H16NO [M+H]+ 178.12. found 178.14
References:
CHIRALGEN, LTD.;Wada, Takeshi;Shimizu, Mamoru US2013/178612, 2013, A1 Location in patent:Paragraph 0091; 0092; 0093
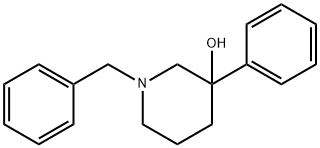
58879-07-5
1 suppliers
inquiry

23396-50-1
46 suppliers
$21.00/100mg
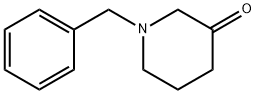
40114-49-6
162 suppliers
$90.00/10g

23396-50-1
46 suppliers
$21.00/100mg
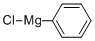
100-59-4
269 suppliers
$38.00/0.25mole

23396-50-1
46 suppliers
$21.00/100mg
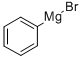
100-58-3
195 suppliers
$18.00/10ml

23396-50-1
46 suppliers
$21.00/100mg