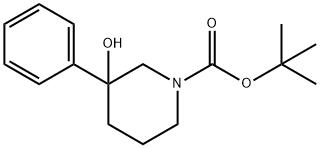
1-N-BOC-3-HYDROXY-3-PHENYLPIPERIDINE synthesis
- Product Name:1-N-BOC-3-HYDROXY-3-PHENYLPIPERIDINE
- CAS Number:213923-81-0
- Molecular formula:C16H23NO3
- Molecular Weight:277.36
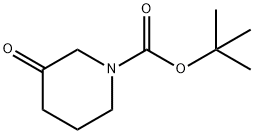
98977-36-7
413 suppliers
$6.00/1g
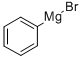
100-58-3
195 suppliers
$18.00/10ml

213923-81-0
31 suppliers
$95.00/100mg
Yield:213923-81-0 34%
Reaction Conditions:
in tetrahydrofuran at -30 - 20; for 12.5 h;
Steps:
1.c (c)
N-Boc-3-Phenylpiperidin-3-ol (3)
(c)
N-Boc-3-Phenylpiperidin-3-ol (3)
N-Boc-3-Pipelidone (1 g, 5 mmol) was dried by azeotropy with dry toluene, and dissolved in dry tetrahydrofuran (THF, 10 ml).
1.08 M Phenyl magnesium bromide in tetrahydrofuran (6.9 ml, 7.5 mmol) was carefully added to the solution at -30° C., and the mixture was stirred for 30 min at -30° C.
The reaction mixture was then gradually warmed to room temperature, and stirring was continued for 12 h.
A mixture of concentrated aqueous ammonia and saturated aqueous ammonium chloride (30 ml, 1:2, v/v) was added to the reaction mixture at 0° C., and extracted with dichloromethane (15 mL, 3 times) and the combined organic layers were dried over sodium sulfate, filtered, and concentrated under reduced pressure.
The residue was chromatographed on silica gel (90 g) using a gradient of methanol (0-2%) in dichloromethane to give Compound 3 (469 mg, 34%) as light yellow oil.
1H NMR (300 MHz, CD3OD) δ 7.53 (2H, d, J=6.9 Hz), 7.34 (2H, t, J=6.9 Hz), 7.25 (1H, d, J=6.9 Hz), 4.20-3.88 (1H, m), 3.88-3.71 (1H, m), 3.42-3.18 (1H, m), 3.08-2.93 (1H, m), 2.13-1.77 (3H, m), 1.58-1.47 (1H, m), 1.46 (9H, s); 13C NMR (75.5 MHz, CD3OD) δ 157.2, 147.5, 129.2, 128.1, 126.3, 81.0, 79.5, 72.4, 37.8, 28.7, 22.3
References:
US2013/178612,2013,A1 Location in patent:Paragraph 0088; 0089; 0090

108-86-1
523 suppliers
$10.00/5g

98977-36-7
413 suppliers
$6.00/1g

213923-81-0
31 suppliers
$95.00/100mg