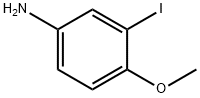
3-IODO-4-METHOXYANILINE synthesis
- Product Name:3-IODO-4-METHOXYANILINE
- CAS Number:74587-12-5
- Molecular formula:C7H8INO
- Molecular Weight:249.05
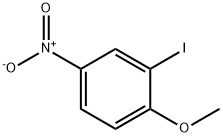
5399-03-1

74587-12-5
General procedure for the synthesis of 3-iodo-4-methoxyaniline from 2-iodo-4-nitroanisole: To a 250 mL round-bottomed flask were added 2-iodo-1-methoxy-4-nitrobenzene (6 g, 21.50 mmol, 1.00 eq.), iron powder (3.61 g, 64.50 mmol, 3.00 eq.), ammonium chloride (3.42 g, 63.94 mmol , 3.00 equiv), ethanol (50 mL) and water (10 mL). The reaction mixture was stirred at 85 °C for 1 hour. Upon completion of the reaction, the solid insoluble material was removed by filtration and the filtrate was concentrated under reduced pressure. The brown solid product 3-iodo-4-methoxyaniline (5.35 g, 100% yield) was obtained. The product was analyzed by liquid chromatography-mass spectrometry (LC-MS): (electrospray ionization, m/z) retention time (RT) = 0.847 min, LCMS 53: m/z = 250 [M + 1].

5399-03-1
136 suppliers
$8.00/1g

74587-12-5
126 suppliers
$9.00/1g
Yield: 100%
Reaction Conditions:
with iron;ammonium chloride in ethanol;water at 85; for 1 h;
Steps:
1.1 Step 1 : Synthesis of 3-iodo-4-methoxyaniline:
Into a 250-mL round-bottom flask was placed 2-iodo-l -methoxy-4-nitrobenzene (6 g, 21 ,50 mmol, 1.00 equiv), Fe (3.61 g, 3.00 equiv), NH4C1 (3.42 g, 63.94 nimol, 3 ,00 equiv), ethanol (50 mL), and water (10 mL). The resulting solution was stirred for 1 h at 85 °C. The solid were filtered out, and the resulting mixture was concentrated under vacuum. This resulted in 5 ,35 g (100%) of the title compound as a brown solid. LC-MS: (ES, m/z) RT = 0.847 min, LCMS 53 : m/z = 250 [M+1].
References:
EPIZYME, INC.;CAMPBELL, John Emmerson;DUNCAN, Kenneth William WO2018/118842, 2018, A1 Location in patent:Paragraph 0421-0424
529-28-2
242 suppliers
$8.00/5g

74587-12-5
126 suppliers
$9.00/1g
104-94-9
497 suppliers
$9.00/1g

74587-12-5
126 suppliers
$9.00/1g
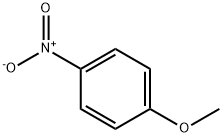
100-17-4
13 suppliers
$22.00/25g

74587-12-5
126 suppliers
$9.00/1g
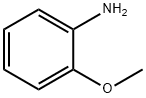
90-04-0
377 suppliers
$5.00/5 g

74587-12-5
126 suppliers
$9.00/1g