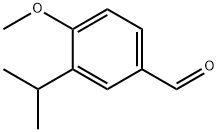
3-ISOPROPYL-4-METHOXYBENZOALDEHYDE synthesis
- Product Name:3-ISOPROPYL-4-METHOXYBENZOALDEHYDE
- CAS Number:31825-29-3
- Molecular formula:C11H14O2
- Molecular Weight:178.23
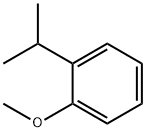
2944-47-0

31825-29-3
Example 2 Preparation of 3-isopropyl-4-methoxybenzaldehyde: A mechanical stirrer was assembled in a 5L three-necked round-bottomed flask and an inert gas protective atmosphere was established. To the reaction flask was added 1-isopropyl-2-methoxybenzene (859 g, 5.85 mol) and dimethylformamide (1584 mL, 20.46 mol), and the mixture was heated to 80 °C. Phosphoryl chloride (2690 g, 17.54 mol) was then added slowly and dropwise. The reaction temperature was maintained between 80 °C and 90 °C for about 3 hours. Immediately after the addition of POCl3, the reaction mixture underwent a color change and gradually deepened with time. The reaction system was continuously stirred at 80 °C for about 16 h, after which the reaction progress was monitored by HPLC to confirm the complete conversion of the feedstock. Upon completion of the reaction, one of two quenching methods was used: method A, slow addition of ice water (8 kg total, containing 5 kg of ice) over about 2 h; method B, dropwise addition of the reaction mixture to deionized water (10 L) in a 30 L jacketed reactor pre-cooled to 0-5 °C over about 3 h. The reaction mixture was then quenched by HPLC. After quenching, ethyl acetate (12 L) was added to the mixture with stirring. After standing and layering, the organic phase was separated and the aqueous phase was extracted with additional ethyl acetate (4L). The combined organic layers were washed sequentially with saturated aqueous sodium bicarbonate (4 L) and saturated aqueous sodium chloride (4 L). Finally, the ethyl acetate was removed by distillation under reduced pressure to afford the target product 3-isopropyl-4-methoxybenzaldehyde (881 g, 86.5% yield).
4885-02-3
200 suppliers
$10.00/1g

2944-47-0
68 suppliers
$12.00/5g

31825-29-3
55 suppliers
$76.00/100mg
Yield:31825-29-3 95%
Reaction Conditions:
with tin(IV) chloride in dichloromethane at 0; for 1 h;
References:
Katoh, Takahiro;Akagi, Taichi;Noguchi, Chie;Kajimoto, Tetsuya;Node, Manabu;Tanaka, Reiko;Nishizawa (nee Iwamoto), Manabu;Ohtsu, Hironori;Suzuki, Noriyuki;Saito, Koichi [Bioorganic and Medicinal Chemistry,2007,vol. 15,# 7,p. 2736 - 2748]

2944-47-0
68 suppliers
$12.00/5g

31825-29-3
55 suppliers
$76.00/100mg

2944-47-0
68 suppliers
$12.00/5g
76-05-1
724 suppliers
$9.00/1g

31825-29-3
55 suppliers
$76.00/100mg
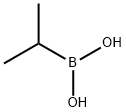
80041-89-0
238 suppliers
$13.50/1G

157790-73-3
0 suppliers
inquiry

31825-29-3
55 suppliers
$76.00/100mg
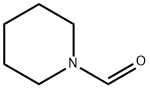
2591-86-8
297 suppliers
$14.00/5g

24591-33-1
14 suppliers
inquiry

31825-29-3
55 suppliers
$76.00/100mg