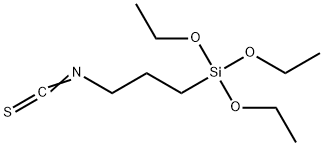
3-isothiocyanatopropyltriethoxysilane synthesis
- Product Name:3-isothiocyanatopropyltriethoxysilane
- CAS Number:58698-89-8
- Molecular formula:C10H21NO3SSi
- Molecular Weight:263.43
Yield: 57%
Reaction Conditions:
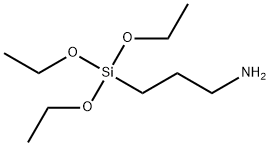
Stage #1:3-aminopropyltriethoxysilane with triethylamine in tetrahydrofuran at 0; for 0.25 h;Inert atmosphere;
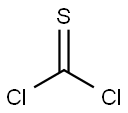
Stage #2:thiophosgene in tetrahydrofuran at 0; for 3 h;Inert atmosphere;
Steps:
Synthesis of triethoxy (3-isothiocyanatopropyl)silane (1)
Triethoxy(3-aminopropyl)silane (APTES) (0.8 g; 3.61 mmol) was added to the flask containing freshly distilled dry THF (15 mL) and dry NEt3(1.1 g; 10.82 mmol) asthe base under nitrogen atmosphere at 0 °C. The reaction mixture was stirred for15 min at this temperature. Then, thiophosgene (0.62 g; 5.42 mmol) was added dropwise through a dropping funnel to the stirring solution at 0 °C under nitrogen atmosphere.The resulting reaction mixture was stirred for further 3 h. At the end of thisperiod, the reaction was allowed to warm to room temperature and filtered off. The filtrate was evaporated under reduced pressure to dryness. Then, the remaining crude product was extracted with water and dichloromethane solvent system. The organic layer was dried over anhydrous Na2SO4,and then the solvent was evaporated underreduced pressure to give a crude product. The obtained crude product was purifiedby silica gel column chromatography. The elution was carried out successively withhexane-ethyl acetate (5:95) solvent system, affording the product as pale-yellow oil.Yield: 0.542 g (57%). FT-IR (disk) ν = 2974, 2927, 2886, 2178, 2097, 1679, 1443,1390, 1345, 1192, 1165, 1099, 1071, 954, 873, 779 cm-1. 1H-NMR (400 MHz,CDCl3,ppm) δ: 0.71-0.75 (m, 2H), 1.26 (t, J = 6.8 Hz, 9H), 1.81-1.89 (m, 2H), 3.54(t, J = 7.0 Hz, 2H), 3.86 (q, J = 6.9 Hz, 6H). 13C-NMR (100 MHz, CDCl3,ppm) δ:7.7 (CH2), 18.3 (CH3), 24.1 (CH2), 47.3 (CH2), 58.5 (CH2), 129.3 (C).
References:
Gök, Yaşar;Gök, Halil Zeki [Research on Chemical Intermediates,2021,vol. 47,# 2,p. 853 - 874]
919-30-2
569 suppliers
$5.00/25g

58698-89-8
18 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)