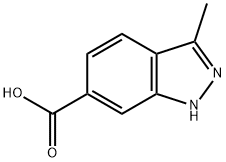
3-Methyl-1H-indazole-6-carboxylic acid synthesis
- Product Name:3-Methyl-1H-indazole-6-carboxylic acid
- CAS Number:201286-96-6
- Molecular formula:C9H8N2O2
- Molecular Weight:176.17
![1H-Indazole-6-carboxylic acid, 3-methyl-1-[(trifluoromethyl)sulfonyl]-, methyl ester](/CAS/20210305/GIF/201286-97-7.gif)
201286-97-7
0 suppliers
inquiry

201286-96-6
39 suppliers
$119.00/500mg
Yield:201286-96-6 80%
Reaction Conditions:
Stage #1: methyl 3-methyl-1-[(trifluoromethyl)sulfonyl]-1H-indazole-6-carboxylatewith water;potassium carbonate in methanol; for 2 h;Heating / reflux;
Stage #2: with potassium hydrogensulfate in water; pH=3 - 3.5;
Steps:
7
A solution of 2-fluoro-4-methoxyacetophenone (2.0 g, 12 mmol) in hydrazine (30 mL) was heated at reflux for 2 days. The mixture was cooled to room temperature, poured into water and extracted with EtOAc (3x). The combined organic extracts were concentrated, dissolved in a minimum amount of CH2CI2, filtered to provide 6-methoxy-3-methyl-1 H-indazole as a yellow solid (620 mg, 32%).To a solution of 6-methoxy-3-methyl-1 H-indazole (620 mg, 3.82 mol) in CH2CI2 (25 mL) at 0 0C was added a dichloromethane solution of boron tribromide (17 mL of 1 M solution). The mixture was stirred at room temperature overnight. The solution was carefully quenched by pouring slowly into iced saturated aqueous NaHCO3. The phases were separated and the aqueous phase was extracted with EtOAc (3x). The combined organic extracts were concentrated and the crude material was purified by Biotage chromatography (4OS column, acetone/heptane 45% 500 mL and 60% 150 mL) to provide 3-methyl-1H- . indazoJr6-ol as an orange, solid (458 mg, 81%).A solution of 3-methyl-1 H-indazol-6:l (458 mg73.1 "mmol) in THF (30 mL) was treated with sodium hydride (0.50 g of 60% oil dispersion). After the initial effervescence had subsided, the solution was heated to 50 0C for 1 hour before cooling to room temperature and adding N-phenyltrifluoromethane- sulphonimide (2.50 g, 7.0 mmol). The mixture was stirred at room temperature for 2 hours before pouring " into water.TheraqueTjus phase was extracted with EtOAc (3x). The combined organic extracts were concentrated and the crude material was purified by Biotage chromatography (4OM column, 12% acetone/heptane). To provide 3-methyl-1-(trifluoromethylsulfonyi)-1H-indazol-6-yl trifluoromethanesulfonate (1.13 g, 89%).A solution of 3-methyl-1-(trifluoromethylsulfonyl)-1 H-indazol-6-yl trifluoromethanesulfonate (0.61 g, 1.5 mmol) in DMF (6 mL) was flushed with carbon dioxide for 5 minutes. To this was added palladium acetate (68 mg, 0.30 mmol), 1,1 -bis(diphenylphosino)ferrocene (167 mg, 0.30 mmol), triethylamine (0.33 g, 0.45 mL, 3.2 mmol), and methanol (4 mL). The solution was stirred at room temperature overnight under one atmosphere of CO. The solution was poured into water and extracted with EtOAc (3x). The combined organic extracts were concentrated and purified by Biotage chromatography (4OS column, 8% EtOAc/heptanes) to provide methyl 3-methyl-1-(trifluoromethylsulfonyl)-1 H-indazole-6-carboxylate (330 mg, 69%).
References:
WO2008/65508,2008,A1 Location in patent:Page/Page column 30-31
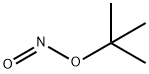
540-80-7
383 suppliers
$29.00/25g
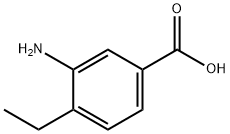
5129-23-7
72 suppliers
$24.00/250mg

201286-96-6
39 suppliers
$119.00/500mg
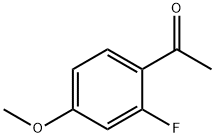
74457-86-6
261 suppliers
$6.00/5g

201286-96-6
39 suppliers
$119.00/500mg
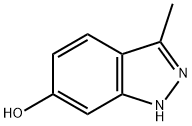
201286-99-9
82 suppliers
$9.00/100mg

201286-96-6
39 suppliers
$119.00/500mg
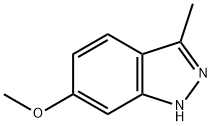
7746-29-4
77 suppliers
$26.00/250mg

201286-96-6
39 suppliers
$119.00/500mg