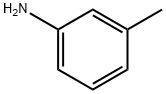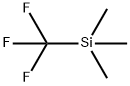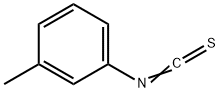
3-Methylphenyl isothiocyanate synthesis
- Product Name:3-Methylphenyl isothiocyanate
- CAS Number:621-30-7
- Molecular formula:C8H7NS
- Molecular Weight:149.21
Yield: 91%
Reaction Conditions:
with phenyllithium in tetrahydrofuran;diethyl ether at -40; for 0.000615 h;Flow reactor;
Steps:
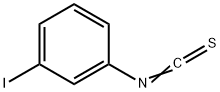
5. Halogen-Li exchange reaction of m-, p-iodophenyl isothiocyanate with PhLiand reaction with various electrophiles using flow microreactors
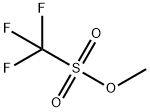
General procedure: A microfluidic system consisting of two T-shaped micromixers (M1 and M2), two tube reactors (R1 and R2) andthree tube pre-temperature-retaining units (for P1, P2 and P3, 1 mm of inner diameter () and 100 cm of length(L)) were used. The microfluidic system was immersed in a temperature control bath. A solution of p-halophenylisothiocyanate or m-halophenyl isothiocyanate (0.1 M in THF) and a solution of phenyllithium reagents (0.44 Min diethyl ether) were individually introduced to M1 (: 250 μm) by syringe pumps. The resulting solution waspassed through R1 ( = 250 μm, L= 3.5 cm) and was mixed with a solution of electrophiles (0.3 M in THF) in M2(: 500 μm). The electrophiles were used as methyl triflate, trimethylsilyl triflate, methyl chloroformate, diethyloxalate, tributylstannyl chloride and NFSI. The resulting solution was passed through R2 (: 1 mm, L: 50 cm).After a steady state was reached, the product solution was collected for 30 s while being quenched with saturatedaqueous NH4Cl solution (2 mL).
References:
Lee, Hyune-Jea;Torii, Daiki;Jeon, Yongju;Yoshida, Jun-Ichi;Kim, Heejin [Synlett,2020,vol. 31,# 19,p. 1899 - 1902] Location in patent:supporting information
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

621-30-7
120 suppliers
$27.00/5g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

621-30-7
120 suppliers
$27.00/5g