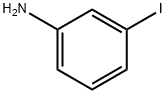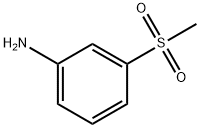
3-(METHYLSULFONYL)ANILINE synthesis
- Product Name:3-(METHYLSULFONYL)ANILINE
- CAS Number:35216-39-8
- Molecular formula:C7H9NO2S
- Molecular Weight:171.22
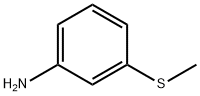
1783-81-9

35216-39-8
General procedure for the synthesis of 3-methylsulfonylaniline from 3-aminothioanisole: A mixture of Na2WO4 (0.067 g), 1 drop of acetic acid and H2O (5 mL) was added to a flask and heated to 65 °C. Subsequently, 3-methylthioaniline (500 mg, 3.59 mmol) was added and H2O2 (1.1 mL, 10.77 mmol) was added slowly and dropwise. The reaction mixture was stirred at 65 °C for 1.5 hours. After completion of the reaction, it was cooled to room temperature and extracted by adding 1N HCl (80 mL) and DCM (50 mL). The organic and aqueous phases were separated and the aqueous phase was then washed with DCM. The aqueous phase was alkalized with 25% NaOH solution and extracted with DCM. The organic phases were combined, washed with brine and dried over Na2SO4. The solvent was removed under reduced pressure to give the 3-methylsulfonylaniline derivative.Preparation of 3-(methylsulfonyl)aniline (158): Compound 158 was prepared from 3-methylsulfonylaniline (153) according to Method E. 557 mg of brown powder was obtained in 92% yield.1H NMR (500 MHz, CDCl3) δ 3.03 (s, 3H), 6.89 (ddd, J = 0.8, 2.3, 8.0 Hz, 1H), 7.21 (t, J = 2.1 Hz, 1H), 7.24 (dt, J = 1.1, 7.7 Hz, 1H), 7.30 (t, J = 7.8 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 44.40, 112.69, 116.37, 119.72, 130.30, 141.16, 141.16. 130.30, 141.16, 147.69. LC-MS (M+H+) calculated value C7H9NO2S 172, measured value 172.

1783-81-9
198 suppliers
$20.00/25g

35216-39-8
75 suppliers
$19.00/100mg
Yield: 92%
Reaction Conditions:
with sodium tungstate;dihydrogen peroxide;acetic acid in water at 65; for 1.5 h;
Steps:
E Oxidation of methylthio aniline to methylsulfonyl aniline
General procedure: A mixture of Na2W04 (0.067 g), 1 drop of acetic acid, and H20 (5 mL) was placed in a flask and heated to 65 °C. Methylthioaniline 153, 154, or 161 (500 mg, 3.59 mmol) was added, followed by dropwise addition of H202 (1.1 mL, 10.77 mmol). The mixture was stirred at 65 °C for 1.5 h and, after cooling, 80 mL of 1 N HC1 and 50 mL of DCM were added. The layers were separated, and the aqueous phase was washed with additional DCM. The aqueous phase was basified with 25% NaOH and extracted with DCM. The organic phase was washed with brine and dried over Na2S04. The solvent was removed to give methylsulfonyl aniline derivatives 157, 158 and 164. [0232] 3-(methylsulfonyl)aniline (158). Compound 158 was prepared according to procedure E from 153 to afford 557 mg of brown powder (92%). 1H NMR (500 MHz, CDC13) δ 3.03 (s, 3H), 6.89 (ddd, J = 0.8, 2.3, 8.0 Hz, 1H), 7.21 (t, J = 2.1Hz, 1H), 7.24 (dt, J = 1.1, 7.7Hz, 1H), 7.30 (t, 7.8Hz, 1H). 13C NMR (126 MHz, CDC13) δ 44.40, 112.69, 116.37, 119.72, 130.30, 141.16, 147.69. LCQ (M+H+) calcd C7H9N02S 172, found 172.
References:
NORTHWESTERN UNIVERSITY;SILVERMAN, Richard, B.;WANG, Hua;KHANFAR, Mohammad WO2014/100833, 2014, A1 Location in patent:Paragraph 0080; 0232
2976-32-1
49 suppliers
$90.00/500mg

35216-39-8
75 suppliers
$19.00/100mg
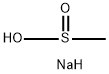
20277-69-4
282 suppliers
$10.00/1g
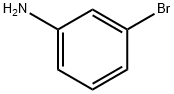
591-19-5
334 suppliers
$10.00/10g

35216-39-8
75 suppliers
$19.00/100mg
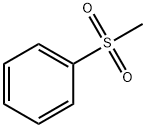
3112-85-4
208 suppliers
$15.00/5g

35216-39-8
75 suppliers
$19.00/100mg