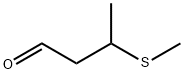
3-(Methylthio)butanal synthesis
- Product Name:3-(Methylthio)butanal
- CAS Number:16630-52-7
- Molecular formula:C5H10OS
- Molecular Weight:118.2

123-73-9
233 suppliers
$40.00/5g
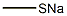
5188-07-8
298 suppliers
$13.00/5g

16630-52-7
225 suppliers
$46.10/10g
Yield: 95%
Reaction Conditions:
with propionic acid in water;toluene at 2 - 70;Product distribution / selectivity;Inert atmosphere;
Steps:
4
To a three-neck 100 mL round bottom flask equipped with a temperature probe, magnetic stirring, and bleach scrubber was charged in sequence 4.67 g (63.04 mmol) of propionic acid followed by 3.60 g (51.36 mmol) of crotonaldehyde followed by 10 mL of toluene, and the mixture was then cooled in an ice-water bath. To this mixture was con-added 25 g (53.50 mmol) of a 15 wt % sodium thiomethoxide in water solution over a 31 min period. The internal reaction temperature rose from 2° C. to 8° C. during the sodium thiomethoxide addition. The ice-water bath was removed and the reaction mixture was allowed to warm to ambient temperature and stirred for an additional 14 min. The reaction mixture was then heated at 50-70° C. for 4 h at which time GC indicated the reaction was complete. After cooling to ambient temperature, the organic layer was separated. The aqueous layer was extracted with 2.5 mL of fresh toluene. The combined organic layers weighed 16.68 g. GC assay of this mixture (using dipropyl phthalate as an internal standard) indicated a 3-thiomethyl butyraldehyde in-pot yield of 95%.
References:
DOW AGROSCIENCES LLC US2010/48939, 2010, A1 Location in patent:Page/Page column 3
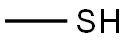
74-93-1
143 suppliers
$454.02/5MG

123-73-9
233 suppliers
$40.00/5g

16630-52-7
225 suppliers
$46.10/10g

123-73-9
233 suppliers
$40.00/5g
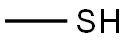
74-93-1
143 suppliers
$454.02/5MG

16630-52-7
225 suppliers
$46.10/10g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry