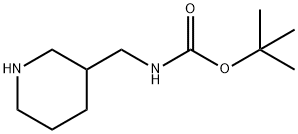
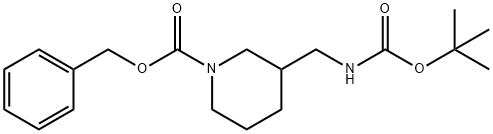
3-N-Boc-Aminomethylpiperidine synthesis
- Product Name:3-N-Boc-Aminomethylpiperidine
- CAS Number:142643-29-6
- Molecular formula:C11H22N2O2
- Molecular Weight:214.3
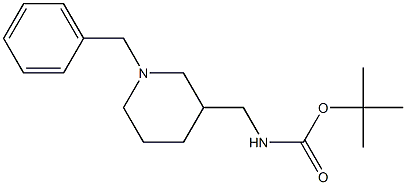
220031-61-8

142643-29-6
To a solution of tert-butyl (1-benzylpiperidin-3-yl)methylcarbamate (2.05 g, 6.74 mmol, 1.0 equiv) in methanol (40 mL) was added 10% Pd(OH)2/C (0.21 g, 10 wt%). Cyclohexene (6.80 mL, 67.40 mmol, 10.0 eq.) was added to the reaction system under argon protection. The resulting suspension was refluxed under argon atmosphere for 20 hours. Upon completion of the reaction, it was filtered through a Celite pad (Celite 577 fine) and the filtrate was concentrated under reduced pressure to give the crude product. The crude product was purified by fast column chromatography with the eluent of dichloromethane/methanol/triethylamine (3:1:0.1, v/v/v) to afford 1.31 g of the target compound 3-Boc-aminomethylpiperidine as a colorless viscous oil. Yield: 91%; Thin Layer Chromatography Rf = 0.33 (dichloromethane/methanol/triethylamine = 3:1:0.1); 1H NMR (400 MHz, CDCl3) δ: 1.08 (qd, J = 11.2, 4.0 Hz, 1H, CH2CHAHBCH), 1.42 (s, 9H, 3 x CH3), 1.45-1.52 (m, 1H. CHAHBCH2CH), 1.58-1.70 (m, 2H, CHAHBCHAHBCH), 1.76-1.82 (m, 1H, CH), 2.32 (t, J = 11.2 Hz, 1H, NHCHAHBCH), 2.52-2.60 (m, 2H, CH2CHAHBNH), 2.97-3.03 (m, 3H, CH2NHCOHBCH), 2.97-3.03 (m, 3H, CH2NHCOHBCH), 2.97-3.03 (m, 3H, CH2NHCO 3H, CH2NHCO + CH2CHAHBNH), 3.06 (dd, J = 11.6, 3.2 Hz, 1H, NHCHAHBCH), 4.63 (br s, 1H, NHCO); 13C NMR (100 MHz, CDCl3) δ: 24.99, 27.97, 28.27, 36.86, 43.74, 45.90, 49.54, 78.28, 155.86; ESI-HRMS m/z calculated C11H23N2O2 [M+H]+ 215.1760, measured 215.1763; IR (KBr) υ: 3385, 2978, 2934, 1685, 1528, 1458, 1367, 1272, 1254, 1174, 1009 cm-1 1174, 1009 cm-1.

220031-61-8
3 suppliers
inquiry

142643-29-6
160 suppliers
$6.00/100mg
Yield:142643-29-6 91%
Reaction Conditions:
with 10% palladium hydroxide on charcoal;cyclohexene in methanol for 20 h;Inert atmosphere;Reflux;
Steps:
Tert-butyl piperidin-3-ylmethylcarbamate (4a)
To a solution of tert-butyl (1-benzylpiperidin-3-yl)methylcarbamate (2.05 g, 6.74 mmol, 1.0equiv.) in MeOH (40 mL), 10% Pd(OH)2 on carbon (0.21 g, 10% mass of tert-butyl (1-benzylpiperidin-3-yl)methylcarbamate) and cyclohexene (6.80 mL, 67.40 mmol, 10.0 equiv.) were added under an argon atmosphere. The resulting suspension was refluxed under an argon atmosphere for 20 h, filtered through a pad of Celite (Celite, 577 fine), and evaporated to obtain a crude product, which was purified by flash column chromatography using DCM/MeOH/Et3N = 3/1/0.1 as eluent, to obtain 1.31 g of the compound as a viscous oil. Yield: 91%; colorless oil; Rf = 0.33 (DCM/MeOH/Et3N = 3/1/0.1); 1H NMR (400 MHz,CDCl3): δ 1.08 (qd, J = 11.2, 4.0 Hz, 1H, CH2CHAHBCH), 1.42 (s, 9H, 3 × CH3), 1.45-1.52(m, 1H, CHAHBCH2CH), 1.58-1.70 (m, 2H, CHAHBCHAHBCH), 1.76-1.82 (m, 1H, CH), 2.32(t, J = 11.2 Hz, 1H, NHCHAHBCH), 2.52-2.60 (m, 2H, CH2CHAHBNH), 2.97-3.03 (m, 3H,CH2NHCO + CH2CHAHBNH), 3.06 (dd, J = 11.6, 3.2 Hz, 1H, NHCHAHBCH), 4.63 (br s, 1H,NHCO); 13C NMR (100 MHz, CDCl3): δ 24.99, 27.97, 28.27, 36.86, 43.74, 45.90, 49.54,S478.28, 155.86; ESI-HRMS m/z calcd. for C11H23N2O2 [M+(H)]+ 215.1760, found 215.1763;IR (KBr) υ = 3385, 2978, 2934, 1685, 1528, 1458, 1367, 1272, 1254, 1174, 1009 cm-1.
References:
Knez, Damijan;Brus, Boris;Coquelle, Nicolas;Sosič, Izidor;Šink, Roman;Brazzolotto, Xavier;Mravljak, Janez;Colletier, Jacques-Philippe;Gobec, Stanislav [Bioorganic and Medicinal Chemistry,2015,vol. 23,# 15,p. 4442 - 4452] Location in patent:supporting information
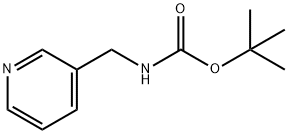
102297-41-6
60 suppliers
$35.75/1g

142643-29-6
160 suppliers
$6.00/100mg
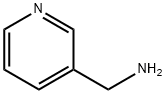
3731-52-0
345 suppliers
$5.00/5g

142643-29-6
160 suppliers
$6.00/100mg

24424-99-5
871 suppliers
$13.50/25G

142643-29-6
160 suppliers
$6.00/100mg
220031-84-5
2 suppliers
inquiry

142643-29-6
160 suppliers
$6.00/100mg