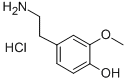
3-O-Methyldopamine hydrochloride synthesis
- Product Name:3-O-Methyldopamine hydrochloride
- CAS Number:1477-68-5
- Molecular formula:C9H13NO2.ClH
- Molecular Weight:203.67
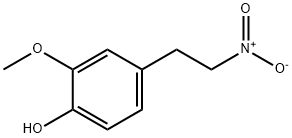
528594-30-1

1477-68-5
General procedure for the synthesis of 4-(2-aminoethyl)-2-methoxyphenol hydrochloride from 2-methoxy-4-(2-nitroethyl)phenol: 2-methoxy-4-(2-nitroethyl)phenol (8 g, 40.6 mmol), ethyl acetate, and concentrated hydrochloric acid were added to a reaction flask and heated to 70 °C. Activated zinc powder (35.0 g, 546.5 mmol) was slowly added under vigorous stirring, followed by slow cooling to 50 °C. After the addition of zinc powder, the reaction was continued for 3 h, during which vigorous stirring was maintained and the reaction progress was monitored by TLC. After completion of the reaction, it was cooled to room temperature and filtered to remove the excess zinc powder. The filtrate was dried with anhydrous sodium sulfate, and the filtrate was collected after filtration again and concentrated to give the oily product 4-(2-aminoethyl)-2-methoxyphenol (6.7 g, 40.0 mmol). To the oily product was added 4 M hydrochloric acid-methanol solution, shaken well and then frozen at -20 °C for 2 hours. Filtration gave the precipitated solid, 3-methoxy-4-hydroxyphenethylamine hydrochloride (6 g, yield about 72%).

528594-30-1
59 suppliers
inquiry

1477-68-5
196 suppliers
$25.00/50mg
Yield:1477-68-5 72%
Reaction Conditions:
Stage #1:2-methoxy-4-(2-nitroethyl)phenol with hydrogenchloride;zinc in water;ethyl acetate at 50 - 70; for 3 h;
Stage #2: with hydrogenchloride in methanol at -20; for 2 h;
Steps:
1.1 Step 1: Preparation of the key intermediate D (3-methoxy-4-hydroxyphenethylamine hydrochloride)
Obtained in the previous step C (8g, 40.6mmol) was added ethyl acetate, concentrated hydrochloric acid, heated to 70 deg. C, was slowly added with vigorous stirring activated zinc dust (35.0g, 546.5mmol), slow cooled to 50 deg. C after the addition was complete, the reaction for 3h, maintaining vigorous stirring during the reaction, TLC detection, after completion of the reaction was cooled to room temperature, filtered to remove the excess zinc, the filtrate was dried over anhydrous sodium sulfate, and then the filtrate was collected by filtration, and the filtrate concentrated to give an oil 4 (6.7g, 40.0mmol), oil 4 was added 4M / L hydrochloric acid - methanol solution, shook, freezed to -20 deg. C 2h, the precipitated solid was suction filtered to give D (3- methoxy-4-hydroxyphenethylamine hydrochloride) 6g (reaction yield of about: 72%).
References:
Chengdu Bestchiralbio Limited-Liability Company;Li, Dequn CN105481849, 2016, A Location in patent:Paragraph 0251; 0252; 0253; 0254; 0257
6178-42-3
75 suppliers
$70.00/5 / G

1477-68-5
196 suppliers
$25.00/50mg
4468-59-1
76 suppliers
$23.00/250 mg

1477-68-5
196 suppliers
$25.00/50mg
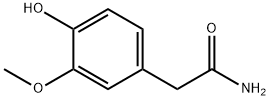
29121-49-1
0 suppliers
inquiry

1477-68-5
196 suppliers
$25.00/50mg
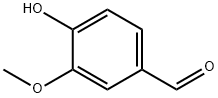
121-33-5
1188 suppliers
$9.00/1g

1477-68-5
196 suppliers
$25.00/50mg