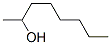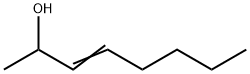
3-OCTEN-2-OL synthesis
- Product Name:3-OCTEN-2-OL
- CAS Number:76649-14-4
- Molecular formula:C8H16O
- Molecular Weight:128.21
Yield:-
Reaction Conditions:
with sodium tetrahydroborate;activated acidic alumina Brockmann II in ethyl acetate at 20; for 1 h;Overall yield = 80 %; Overall yield = 205 mg;
Steps:
4.1.2. General procedure for 1,2-reduction of a,b-unsaturated ketonesto allylic alcohols.
To a reaction vial equipped with a stir bar wereadded: activated acidic alumina Brockmann II (acidic-Al2O3-B2,6 g), EtOAc (10 mL), and the a,b-unsaturated ketone substrate(2 mmol). The vial was capped and the mixturewas stirred at roomtemperature for 10 min before NaBH4 (152 mg, 2.0 mmol) wasadded in one portion. The reaction vial was capped and the mixturewas stirred at room temperature and monitored by TLC and/or 1HNMR until complete disappearance of starting material was observed.The reaction mixture was filtered through filter paper(Whatman, 42 Ashless) and the solids washed with acetone (approximately320 mL). The filtrate was concentrated in vacuo andthe residue purified via flash column chromatography on silica gel(EtOAc/hexanes with gradient elution).
References:
Jones-Mensah, Ebenezer;Nickerson, Leslie A.;Deobald, Jackson L.;Knox, Hailey J.;Ertel, Alyssa B.;Magolan, Jakob [Tetrahedron,2016,vol. 72,# 26,p. 3748 - 3753]
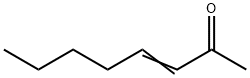
1669-44-9
80 suppliers
$6.00/1g

76649-14-4
28 suppliers
$839.22/5G