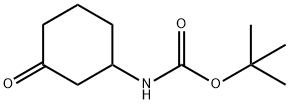
(3-OXO-CYCLOHEXYL)-CARBAMIC ACID TERT-BUTYL ESTER synthesis
- Product Name:(3-OXO-CYCLOHEXYL)-CARBAMIC ACID TERT-BUTYL ESTER
- CAS Number:885280-38-6
- Molecular formula:C11H19NO3
- Molecular Weight:213.27
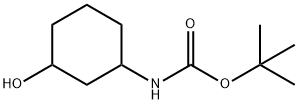
610302-03-9

885280-38-6
General procedure for the synthesis of N-Boc-3-cyclohexanone amine from tert-butyl 3-hydroxycyclohexylcarbamate: To a solution of tert-butyl (3-hydroxycyclohexyl)carbamate (4.40 g, 20.46 mmol) in dichloromethane (DCM, 250 mL) was added Dess-Martin periodinane (13.0 g, 30.70 mmol). The reaction mixture was cooled in an ice bath and then stirred at room temperature for 2 hours. After completion of the reaction, the reaction mixture was diluted with DCM and washed with aqueous sodium carbonate. The organic layer was separated and dried with anhydrous sodium sulfate and subsequently concentrated. The crude product was purified by silica gel column chromatography (eluent: 40% petroleum ether solution of ethyl acetate) to afford N-Boc-3-cyclohexanone amine (4.0 g, 18.78 mmol, 91% yield). Mass spectrum (ESI) m/z 214.1 [M + H]+.

610302-03-9
111 suppliers
$30.00/250mg

885280-38-6
178 suppliers
$8.00/250mg
Yield:885280-38-6 91%
Reaction Conditions:
with Dess-Martin periodane in dichloromethane at 20; for 2 h;Cooling with ice;
Steps:
27.D tert-butyl(3-oxocyclohexyl)carbamate
D.
tert-butyl(3-Oxocyclohexyl)carbamate
To a solution of tert-butyl (3-hydroxycyclohexyl)carbamate (4.40 g, 20.46 mmol) in DCM (250 mL) was added Dess-Martin periodinane (13.0 g, 30.70 mmol) in portions under cooling in an ice bath.
The mixture was stirred at room temperature for 2 h.
The reaction was diluted with DCM and washed with aqueous sodium carbonate.
The separated organic layer was dried over anhydrous sodium sulfate and concentrated.
The crude compound was purified by silica gel column chromatography (40% ethyl acetate in petroleum ether) to afford the title compound (4.0 g, 18.78 mmol, 91% yield). MS (ESI) m/z 214.1 [M+H]+.
References:
Papa, Patrick;Cathers, Brian Edwin;CALABRESE, Andrew Antony;WHITEFIELD, Brandon Wade;BENNETT, Brydon;CASHION, Daniel;MORTENSEN, Deborah;HUANG, Dehua;TORRES, Eduardo;PARNES, Jason;SAPIENZA, John;HANSEN, Joshua;LEFTHERIS, Katerina;CORREA, Matthew;DELGADO, Maria Mercedes;RAHEJA, Neil;BAHMANYAR, Sami;HEGDE, Sayee;NORRIS, Stephen;PLANTEVIN-KRENITSKY, Veronique US2015/175557, 2015, A1 Location in patent:Paragraph 0383
930-68-7
267 suppliers
$9.00/1g
4248-19-5
380 suppliers
$5.00/5g

885280-38-6
178 suppliers
$8.00/250mg
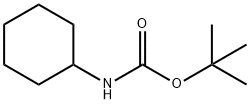
3712-40-1
16 suppliers
$265.00/2.5g

885280-38-6
178 suppliers
$8.00/250mg

24424-99-5
869 suppliers
$13.50/25G

885280-38-6
178 suppliers
$8.00/250mg
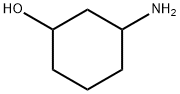
6850-39-1
164 suppliers
$15.00/250mg

885280-38-6
178 suppliers
$8.00/250mg