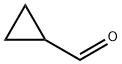
N-Boc-4-(Cyclopropylmethyl)piperazine synthesis
- Product Name:N-Boc-4-(Cyclopropylmethyl)piperazine
- CAS Number:373608-50-5
- Molecular formula:C13H24N2O2
- Molecular Weight:240.34
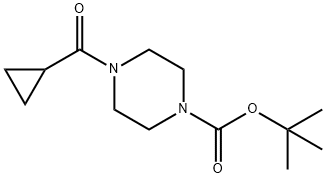
414910-15-9
103 suppliers
$12.00/1mg

373608-50-5
17 suppliers
inquiry
Yield:373608-50-5 94%
Reaction Conditions:
with sodium tetrahydroborate;boron trifluoride diethyl etherate in tetrahydrofuran at 0 - 25; for 4 h;Inert atmosphere;Large scale;Solvent;
Steps:
1.2
S2: 20L four-neck flask with mechanical stirring and thermometer, nitrogen protection,Add tert-butyl 4-(cyclopropanecarbonyl)piperazine-1-carboxylate (2.40 kg, 9.44 mol),Sodium borohydride (0.71 kg, 18.88 mol), THF 5.06 Kg, cooled to 10 ° C,Boron trifluoride etherate (2.02 kg, 14.16 mol) was slowly added dropwise, and the temperature was controlled from 0 ° C to 10 ° C.After the dropwise addition is completed, the reaction is carried out at 10 ° C to 25 ° C for 4 h.The reaction solution was slowly poured into ice water of 5 kg of ice and 5 kg of water, and extracted with methyl tert-butyl ether (4 kg * 2).The methyl tert-butyl ether phase was combined and washed once with 5 kg of saturated sodium chloride solution.Concentration and removal of methyl tert-butyl ether gave a pale-yellow solid, N-Boc-4-(cyclopropylmethyl)piperazine, 2.13 kg, yield 94%.The nuclear magnetic warp alignment is consistent with the standard map.
References:
CN108341792,2018,A Location in patent:Paragraph 0029; 0034

5911-08-0
205 suppliers
$25.00/1g
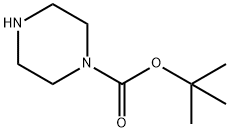
57260-71-6
729 suppliers
$5.00/5g

373608-50-5
17 suppliers
inquiry

57260-71-6
729 suppliers
$5.00/5g

373608-50-5
17 suppliers
inquiry