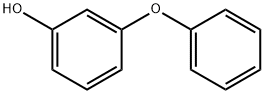
3-PHENOXYPHENOL synthesis
- Product Name:3-PHENOXYPHENOL
- CAS Number:713-68-8
- Molecular formula:C12H10O2
- Molecular Weight:186.21
Yield: 80%
Reaction Conditions:
with copper(I) chloride;8-quinolinol;potassium carbonate in N,N-dimethyl-formamide at 100; for 0.5 h;
Steps:
3-Phenoxyphenol (2)
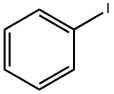
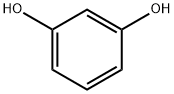
A mixture of 10.2 g (50 mmol)of iodobenzene, 6.6 g (60 mmol) of resorcinol, 10.3 g(70 mmol) of K2CO3, 0.05 g (0.5 mmol) of copper(I)chloride, 0.07 g (0.5 mmol) of 8-hydroxyquinoline,and 60 mL of DMF was stirred for 30 min at 100°,cooled, diluted with 200 mL of water, acidified withsulfuric acid to 2, and extracted with 150 mL ofbenzene. The organic layer was separated, the reactionproduct was extracted with 15% solution of NaOH, thealkaline solution was acidified with sulfuric acid to 1,the separated precipitate was filtered off, dissolved inbenzene and washed with water till negative test forresorcinol (test with FeCl3). The solvent was distilledoff. Yield 7.4 g (80%), light-yellow fluid, nD20 1.6010[13]. 1 NMR spectrum, δ, ppm: 5.45 br.s (1), 6.53 s(1), 6.59-6.62 t (2, J 7.3 Hz), 7.06-7.08 d (2, J8.1 Hz), 7.16-7.17 t (1, J 6.9 Hz), 7.18-7.22 t (1, J7.3 Hz), 7.36-7.39 t (2, J 7.2 Hz).
References:
Koptyaev;Ageeva;Galanin;Shaposhnikov [Russian Journal of Organic Chemistry,2016,vol. 52,# 2,p. 261 - 267][Zh. Org. Khim.,2016,vol. 52,# 2,p. 278 - 283,5]
87199-18-6
302 suppliers
$5.00/250mg

108-95-2
786 suppliers
$14.00/25g

713-68-8
86 suppliers
$130.00/5g
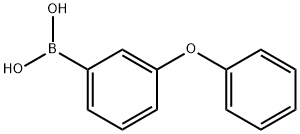
221006-66-2
54 suppliers
$45.00/50mg

713-68-8
86 suppliers
$130.00/5g
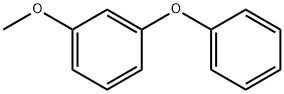
1655-68-1
16 suppliers
$49.50/5g

713-68-8
86 suppliers
$130.00/5g
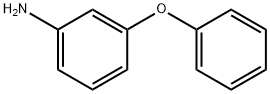
3586-12-7
88 suppliers
$39.60/5g

713-68-8
86 suppliers
$130.00/5g