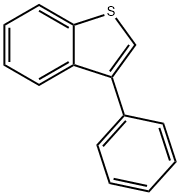
3-Phenylbenzo[b]thiophene synthesis
- Product Name:3-Phenylbenzo[b]thiophene
- CAS Number:14315-12-9
- Molecular formula:C14H10S
- Molecular Weight:210.29
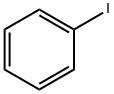
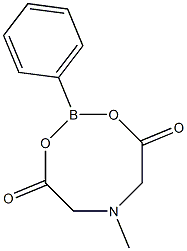
591-50-4
505 suppliers
$10.00/1g
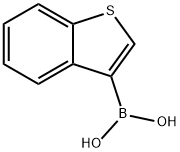
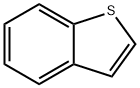
113893-08-6
236 suppliers
$8.00/250mg
![3-Phenylbenzo[b]thiophene](/CAS/GIF/14315-12-9.gif)
14315-12-9
6 suppliers
inquiry
Yield:14315-12-9 100%
Reaction Conditions:
with sodium carbonate;tetrakis(triphenylphosphine) palladium(0) in ethanol;water;toluene; for 6 h;Reflux;
Steps:
3.1
Step 1 : Preparation of intermediate 3-phenyl-1 -benzothiophene (3a)A solution of benzothiopheneboronic acid (1 g, 5.61 mmol) in ethanol (23 mL) was added to a mixture of iodobenzene (560 μΙ_, 5 mmol), palladium tetrakis(triphenylphosphine) (460 mg, 0.4 mmol) and sodium carbonate (2.38 g, 22.4 mmol) in a mixture of toluene (15 mL) and water (15 mL). The reaction mixture was refluxed for 6 hours and then concentrated in vacuo. The residue was partitioned between ethyl acetate (30 mL) and water (10 mL). The biphasic mixture was acidified with an aqueous solution of hydrochloric acid 1 N until pH 2. The aqueous layer was extracted with ethyl acetate (2 x 20 mL) and the organic layer was washed with water (10 mL), brine (10 mL), dried over sodium sulfate, filtered and evaporated to dryness. The residue was purified by flash chromatography on silica gel (cyclohexane) to afford the desired product (3a) as a yellow solid (1 .18 g, 5.61 mmol, 100%).1H NMR (400 MHz, CDCI3) 7.35-7.45 (m, 4H), 7.49 (t, J = 7.6 Hz, 2H), 7.59 (d, J = 7.0 Hz, 2H), 7.89-7.95 (m, 2H).
References:
WO2012/137181,2012,A1 Location in patent:Page/Page column 50
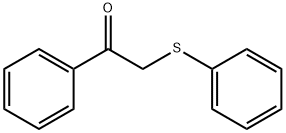
16222-10-9
4 suppliers
inquiry
![3-Phenylbenzo[b]thiophene](/CAS/GIF/14315-12-9.gif)
14315-12-9
6 suppliers
inquiry
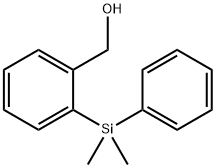
853955-69-8
17 suppliers
$39.00/250mg
7342-82-7
182 suppliers
$5.00/1g
![3-Phenylbenzo[b]thiophene](/CAS/GIF/14315-12-9.gif)
14315-12-9
6 suppliers
inquiry
![1,1-DiMethyl-1,3-dihydrobenzo[c][1,2]oxasilole](/CAS/GIF/321903-29-1.gif)
321903-29-1
38 suppliers
$47.00/100mg