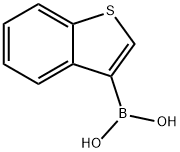
Benzothiophene-3-boronic acid synthesis
- Product Name:Benzothiophene-3-boronic acid
- CAS Number:113893-08-6
- Molecular formula:C8H7BO2S
- Molecular Weight:178.02
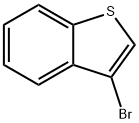
7342-82-7

5419-55-6

113893-08-6
Under nitrogen protection, 10 g (47 mmol) of 3-bromobenzothiophene was dissolved in 100 ml of tetrahydrofuran (THF) and cooled to -60 °C. 36 ml of hexane solution of n-butyllithium (concentration 1.57 mol/L) was slowly added dropwise, and after the dropwise addition, the reaction mixture was continued to be stirred for 1 hour. Subsequently, 13.3 g (71 mmol) of triisopropyl borate was added and stirring was continued for 1 hour. The reaction system was slowly brought back to room temperature and the reaction was quenched by the addition of 50 ml of saturated aqueous ammonium chloride solution. The reaction mixture was extracted with 100 ml of toluene, the organic phase was washed three times (100 ml each) with distilled water and dried over anhydrous magnesium sulfate. The desiccant was removed by filtration and the solvent was removed by concentration under reduced pressure to afford intermediate B-1. 6.9 g (39 mmol, 83% yield) of the target product, benzothiophene-3-boronic acid, was finally obtained.

7342-82-7
191 suppliers
$5.00/1g

5419-55-6
388 suppliers
$12.00/5g

113893-08-6
235 suppliers
$8.00/250mg
Yield:113893-08-6 83%
Reaction Conditions:
Stage #1:3-Bromothianaphthene with n-butyllithium in tetrahydrofuran;hexane at -60; for 1 h;Inert atmosphere;
Stage #2:Triisopropyl borate in tetrahydrofuran;hexane for 1 h;Inert atmosphere;
Steps:
9
Under a nitrogen atmosphere, 100 ml of THF was added to 10 g (47 mmol) of 3-bromobenzothiophene and cooled to - 60°C, 36 ml of a hexane solution of n-butyllithium (1.57 mol/l was added dropwise, and the mixture was stirred for 1 hour. Then, 13.3 g (71 mmol) of triiospropyl borate was added and the stirring was continued for 1 hour. The reaction solution was returned to room temperature and 50 ml of a saturated aqueous solution of ammonium chloride and 100 ml of toluene were added. The organic layer was washed with distilled water (3×100 ml) and dried over anhydrous magnesium sulfate, the magnesium sulfate was separated by filtration, the solvent was distilled off under reduced pressure, and Intermediate B-1 weighing 6.9 g (39 mmol, 83% yield) was obtained
References:
Nippon Steel & Sumikin Chemical Co., Ltd.;HOTTA Masanori;SAWADA Yuichi;KAWADA Atsushi;JIKUMARU Masana;MATSUMOTO Megumi EP2617724, 2013, A1 Location in patent:Paragraph 0162; 0163

7342-82-7
191 suppliers
$5.00/1g

121-43-7
382 suppliers
$13.00/25mL

113893-08-6
235 suppliers
$8.00/250mg

7342-82-7
191 suppliers
$5.00/1g

52732-22-6
0 suppliers
inquiry

113893-08-6
235 suppliers
$8.00/250mg
95-15-8
395 suppliers
$6.00/5g

113893-08-6
235 suppliers
$8.00/250mg