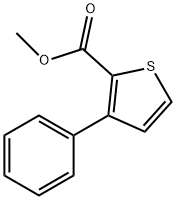
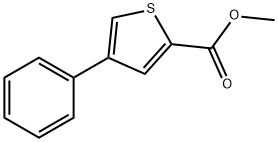
3-Phenylthiophene-2-carboxylic Acid Methyl Ester synthesis
- Product Name:3-Phenylthiophene-2-carboxylic Acid Methyl Ester
- CAS Number:21676-89-1
- Molecular formula:C12H10O2S
- Molecular Weight:218.27
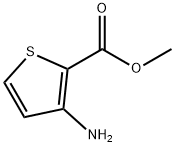
22288-78-4
394 suppliers
$11.00/5g
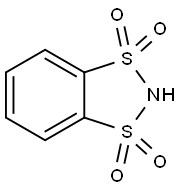
4482-01-3
25 suppliers
$60.00/100mg
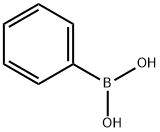
98-80-6
744 suppliers
$5.00/5g

21676-89-1
9 suppliers
$120.00/10mg
Yield:21676-89-1 91%
Reaction Conditions:
Stage #1: Methyl 3-aminothiophene-2-carboxylate;o-benzenedisulfonimidewith acetic acid at 0 - 5; for 0.166667 h;Inert atmosphere;
Stage #2: with isopentyl nitrite for 0.166667 h;Inert atmosphere;
Stage #3: phenylboronic acidwith bis[(trifluoromethanesulfonyl)imidate]-2-(dicyclohexyl(2’,6’-dimethoxybiphenyl))phosphine gold(I);caesium carbonate in tetrahydrofuran at 20; for 3 h;Inert atmosphere;chemoselective reaction;Suzuki-Miyaura Coupling;
Steps:
3.31 4.3 4-Methoxybiphenyl (4a): representative procedure for the Au catalysed Suzuki-Miyaura couplings
General procedure: In a oven-dried flask and under nitrogen flow, benzenediazonium o-benzenedisulfonimide (1a, 161mg, 0.5mmol) was added to a suspension of 4-methoxyphenylboronic acid (3a, 91mg, 0.6mmol), [bis(trifluoromethanesulfonyl)imidate](triphenylphosphine)gold(I) (2:1) toluene adduct (39mg, 0.025mmol, 5mol%), Cs2CO3 (325mg, 1mmol) in THF (5mL). The resulting mixture was stirred at room temperature for 2h; the completion of the reaction was confirmed by the absence of azo coupling with 2-naphthol. Then, the reaction mixture was poured into diethyl ether/water (100mL, 1:1). The aqueous layer was separated and extracted with diethyl ether (50mL). The combined organic extracts were washed with water (50mL), dried with Na2SO4 and evaporated under reduced pressure. GC-MS analyses of the crude residue showed 4-methoxybiphenyl (4a), MS (EI): m/z 184 (M+) as the major product, besides traces of biphenyl, MS (EI): m/z 154 (M+), 4,4'-dimethoxybiphenyl, MS (EI): m/z 214 (M+), N-phenyl-o-benzenedisulfonimide, MS (EI): m/z 295 (M+). The crude residue was purified on a short column, eluting with petroleum ether/diethyl ether (9:1). The only isolated product was the title compound (4a, 81mg, 88% yield).
References:
Barbero, Margherita;Dughera, Stefano [Tetrahedron,2018,vol. 74,# 39,p. 5758 - 5769]
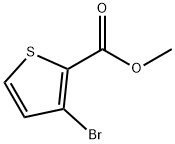
26137-08-6
175 suppliers
$7.00/250mg

98-80-6
744 suppliers
$5.00/5g

21676-89-1
9 suppliers
$120.00/10mg
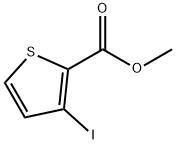
62353-77-9
52 suppliers
$15.00/250mg

98-80-6
744 suppliers
$5.00/5g

21676-89-1
9 suppliers
$120.00/10mg
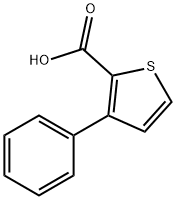
10341-88-5
25 suppliers
$138.00/5MG

74-88-4
356 suppliers
$15.00/10g

21676-89-1
9 suppliers
$120.00/10mg

124-38-9
134 suppliers
$214.00/14L
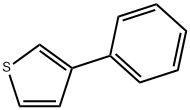
2404-87-7
99 suppliers
$41.00/250mg

74-88-4
356 suppliers
$15.00/10g

21676-89-1
9 suppliers
$120.00/10mg
21676-90-4
31 suppliers
$105.00/1g