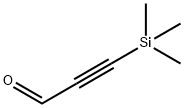
3-TRIMETHYLSILYLPROPYNAL synthesis
- Product Name:3-TRIMETHYLSILYLPROPYNAL
- CAS Number:2975-46-4
- Molecular formula:C6H10OSi
- Molecular Weight:126.23

68-12-2
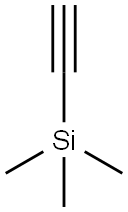
1066-54-2

2975-46-4
Steps for the synthesis of 3-trimethylsilylpropynal: 1. A solution of trimethylsilylacetylene (5.0 mL, 36.10 mmol) in THF (25.0 mL) was added dropwise to a solution of EtMgBr in THF (1 M, 44.0 mL, 44.0 mmol) under nitrogen protection, and the reaction temperature was maintained at 10-150°C. 2. After the dropwise addition, the reaction mixture was stirred at room temperature for 1 hour. 3. A solution of Et2O (20.0 mL) in DMF (10.0 mL, 123.0 mmol) was slowly added to the reaction system at -25 °C, and the addition time was controlled to be within 30 min. 4. The resulting white suspension was gradually warmed to room temperature and stirring was continued for 1 hour. 5. The reaction mixture was heated to 30 °C and maintained for 15 minutes. 6. The reaction solution was cooled to 0 °C and poured into 5% H2SO4 solution. 7. The aqueous layer was separated and extracted three times with Et2O. 8. Combine the organic layers, wash with saturated aqueous NH4Cl solution and dry with anhydrous Na2SO4. 9. Concentrate under pressure to remove the solvent and obtain the crude product. 10. purification of the crude product by ball-to-ball distillation (20 mbar, room temperature) afforded 3-trimethylsilylpropynal (2.255 g, 49% yield). Product characterization data: 1H NMR: δ 9.15 (s, 1H), 0.25 (s, 9H). 13C NMR: δ 176.7, 103.0, 102.3, 0.88.

1066-54-2
478 suppliers
$9.00/10g

2975-46-4
138 suppliers
$19.00/1g
Yield:2975-46-4 28%
Reaction Conditions:
with hydrogenchloride;n-butyllithium in diethyl ether;hexane;N,N-dimethyl-formamide
Steps:
2 Example 2
Example 2 Ethynyltrimethylsilane (5.0 g, 50.9 mmol) was dissolved in dry diethyl ether (50 ml), 1.6 M n-butyllithium in hexane(35.0 ml, 56.0 mmol) was added dropwise at 0°C under argon atmosphere. The mixture was stirred at the same temperature for 1h. DMF(3.72g, 50.9mmol) was dissolved in diethyl ether (20ml), and was added dropwise below 5°C for 30 min, then the mixture was stirred at room temperature for 2 h. The reaction was quenched by the addition of 2N hydrochloric acid, and the mixture was extracted with diethyl ether. The organic layer was washed with water, saturated sodium hydrogencarbonate solution, and brine in order, and dried over sodium sulfate. Purification by distillation (40-45°C/15mmHg) gave 3-(trimethylsilyl)propiol aldehyde (28%). Colorless oil 1H-NMR (CDCl3) δ:0.27 (9H, s), 9.17 (1H, s).
References:
Institute of Medicinal Molecular Design, Inc. EP1145718, 2001, A1
5272-36-6
199 suppliers
$6.00/1g

2975-46-4
138 suppliers
$19.00/1g
42201-71-8
57 suppliers
$45.00/250mg

2975-46-4
138 suppliers
$19.00/1g
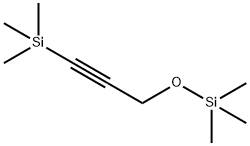
50965-66-7
21 suppliers
inquiry

2975-46-4
138 suppliers
$19.00/1g
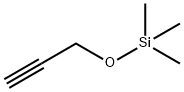
5582-62-7
126 suppliers
$38.79/5g

2975-46-4
138 suppliers
$19.00/1g