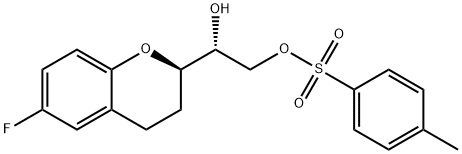
(1’R,2R)-2-(2’-Tosyl-1’,2’-dihydroxyethyl)-6-fluorochromane synthesis
- Product Name:(1’R,2R)-2-(2’-Tosyl-1’,2’-dihydroxyethyl)-6-fluorochromane
- CAS Number:303176-46-7
- Molecular formula:C18H19FO5S
- Molecular Weight:366.4

98-59-9
618 suppliers
$9.00/5g
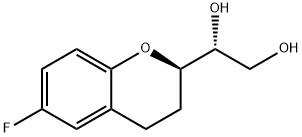
303176-45-6
14 suppliers
$140.00/100mg

303176-46-7
11 suppliers
$115.00/25mg
Yield:303176-46-7 323 g
Reaction Conditions:
with pyridine in dichloromethane at 20; for 48 h;
Steps:
1 Preparation of (R, R)-Compound I and (S, R)-Compound I
(2R) -3,4-dihydro-2H-2-benzopyrane-4-one] - (1R) -1,2-diazabicyclo [ - ethylene glycol,Decarbonylation in trifluoroacetic acid / triethylsilane gave 1- [6-fluoro- (2R) -3,4-dihydro-2H-2-benzopyran] - (1R) -1,2- Ethylene glycol. Adding 240mL of dichloromethane, stirring, adding 520mL pyridine, dissolved immediately clear, ice water bath cooling; also called p-toluenesulfonyl chloride 236g, adding constant pressure funnel, 800mL dichloromethane into the constant pressure funnel, Can be dissolved completely, the formation of suspension, in the ice water bath, the suspension droplets added to the reaction bottle, drop is completed, remove the ice bath, room temperature stirring reaction 2 days, the reaction is completed, the reaction solution into 3L water , Washed with dichloromethane, washed with water 2L X 3 times, dried over anhydrous sodium sulphate and evaporated to dryness to give a white solid which was washed with petroleum ether and dried to give 323 g of (R, R) I.
References:
CN103910704,2016,B Location in patent:Paragraph 0076; 0077
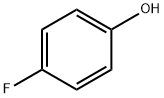
371-41-5
531 suppliers
$8.00/25g

303176-46-7
11 suppliers
$115.00/25mg
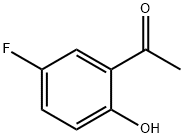
394-32-1
356 suppliers
$9.00/5g

303176-46-7
11 suppliers
$115.00/25mg
![(1’R,2R)-2-[(1’,2’-O-Isopropylidene)dihydroxyethyl]-6-fluorochroman-4-one](/CAS/20180808/GIF/797054-18-3.gif)
797054-18-3
7 suppliers
$140.00/250mg

303176-46-7
11 suppliers
$115.00/25mg
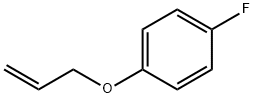
13990-72-2
6 suppliers
inquiry

303176-46-7
11 suppliers
$115.00/25mg