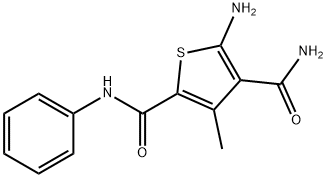
5-amino-3-methyl-N~2~-phenylthiophene-2,4-dicarboxamide synthesis
- Product Name:5-amino-3-methyl-N~2~-phenylthiophene-2,4-dicarboxamide
- CAS Number:331959-40-1
- Molecular formula:C13H13N3O2S
- Molecular Weight:275.33
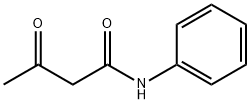
102-01-2
0 suppliers
$5.00/100g
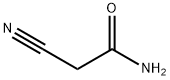
107-91-5
569 suppliers
$5.00/10g

331959-40-1
9 suppliers
$126.00/500mg
Yield:331959-40-1 89%
Reaction Conditions:
with morpholine;sulfur in ethanol at 80;
Steps:
5-Amino-3-methyl-N2-phenylthiophene-2,4-dicarboxamide (11)
To a solution of 2-cyanoacetamide (59mg, 0.70mmol) in anhydrous EtOH (3mL) was added acetoacetanilide (124mg, 0.70mmol) and sulfur (23mg, 0.70mmol), followed by the addition of morpholine (121mg, 1.40mmol) dropwise. The reaction mixture was stirred at 80°C until no starting material left as shown by TLC. Solvent was removed and the residue was purified by flash chromatography on silica gel using hexanes:EtOAc (1:2) to obtain compound 11 (170mg, 89%). Mp 234-235°C 1H NMR (600MHz, DMSO- d6) δ 9.57 (s, 1H), 7.67 (d, J=8.4Hz, 2H), 7.36 (m, 4H), 7.12 (m, 1H), 7.05 (s, 2H), 2.41 (s, 3H); 13C NMR (100MHz, DMSO-d6) δ 167.9, 162.0, 161.3, 140.2, 139.7, 128.9, 123.5, 120.6, 112.4, 112.0, 16.3; C13H14N3O2S [M+H]+ 276.0803, found 276.0801.
References:
Tang, Jing;Huber, Andrew D.;Pineda, Dallas L.;Boschert, Kelsey N.;Wolf, Jennifer J.;Kankanala, Jayakanth;Xie, Jiashu;Sarafianos, Stefan G.;Wang, Zhengqiang [European Journal of Medicinal Chemistry,2019,vol. 164,p. 179 - 192] Location in patent:supporting information

62-53-3
682 suppliers
$10.00/1g

331959-40-1
9 suppliers
$126.00/500mg