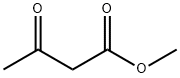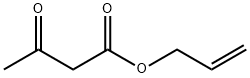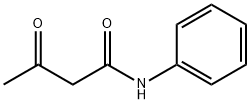
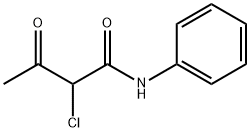
Acetoacetanilide synthesis
- Product Name:Acetoacetanilide
- CAS Number:102-01-2
- Molecular formula:C10H11NO2
- Molecular Weight:177.2
Yield:102-01-2 88%
Reaction Conditions:
with 1,1,1,3',3',3'-hexafluoro-propanol in acetonitrile at 80; for 12 h;Sealed tube;Green chemistry;
Steps:
5. General experimental procedure for the synthesis of β-oxo amides 3aa-va using amines 1a-v and β-keto esters 2a-c.
General procedure: A 10 mL reaction vial was charged with amines 1a-v (1.0 mmol), ethyl acetoacetate (2a) (1.2 mmol) or methyl acetoacetate (2b) (1.2 mmol) or allyl acetoacetate (2c) (1.2 mmol) and HFIP (10 mmol). The reaction vial was then sealed and heated at 80 °C for 12 h. After completion of the reaction (progress was monitored by TLC; SiO2, Hexane/EtOAc = 8:2), the mixture was diluted with ethyl acetate (15 mL) and water (20 mL) and extracted with ethyl acetate (3 × 10 mL). The combined organic layers were washed with brine (3 × 10 mL) and dried over anhydrous Na2SO4. The solvents were removed under reduced pressure and the crude products were purified by column chromatography using silica gel (100-200 mesh) with hexane/EtOAc (8:2) as the eluent to obtain the desired products (3aa-va) in good to excellent yields.
References:
Kabi, Arup K.;Gujjarappa, Raghuram;Vodnala, Nagaraju;Kaldhi, Dhananjaya;Tyagi, Ujjawal;Mukherjee, Kalisadhan;Malakar, Chandi C. [Tetrahedron Letters,2020,vol. 61,# 46,art. no. 152535] Location in patent:supporting information
31844-92-5
29 suppliers
$60.00/500 mg

102-01-2
0 suppliers
$5.00/100g