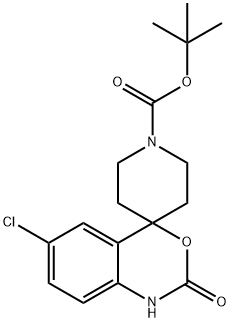
6-CHLORO-1,2-DIHYDRO-2-OXOSPIRO[4H-3,1-BENZOXAZINE-4,4'-PIPERIDINE]-1'-CARBOXYLIC ACID 1,1-DIMETHYL ETHYL ESTER synthesis
- Product Name:6-CHLORO-1,2-DIHYDRO-2-OXOSPIRO[4H-3,1-BENZOXAZINE-4,4'-PIPERIDINE]-1'-CARBOXYLIC ACID 1,1-DIMETHYL ETHYL ESTER
- CAS Number:332187-61-8
- Molecular formula:C17H21ClN2O4
- Molecular Weight:352.81
106-47-8
481 suppliers
$5.00/5G
![6-CHLORO-1,2-DIHYDRO-2-OXOSPIRO[4H-3,1-BENZOXAZINE-4,4'-PIPERIDINE]-1'-CARBOXYLIC ACID 1,1-DIMETHYL ETHYL ESTER](/CAS/GIF/332187-61-8.gif)
332187-61-8
35 suppliers
$140.00/50mg
Yield:-
Steps:
3
6-Chloro-1-[2-(dimethylamino)ethyl]spiro[3, 1-benzoxazine-4,4'-piperidin]-2(1H)-one; To a solution of 0.100 g (0.283 mmol) tert-butyl 6-chloro-2-oxo-1,2-dihydro-1'H-spiro[3,1-benzoxazine-4,4'-piperidine]-1'-carboxylate (described in WO0122919 A2, and prepared also using the procedure described in “J. Med. Chem.,1983, 26(5), 657” starting from 4-chloro aniline) in 7 ml THF was added 0.025 g (0.566 mmol) NaH. After 30 minutes at RT, 0.063 g (0.566 mmol) (2-chloro-ethyl)-dimethyl-amine was added. The reaction mixture was stirred at 60° C. overnight, then poured onto an aqueous solution of NH4Cl and extracted twice with EtOAc. The combined organic phases were dried over Na2SO4 and concentrated in vacuo yielded 60 mg of a white solid. This crude material was then dissolved in 5 ml CH2Cl2 and 1 ml of TFA was added. After 2 hours at RT, the solvent were removed under vacuo and the resulting oil was taken up in CH2Cl2 and washed with an aqueous solution of NaHCO3. The organic phase was dried with Na2SO4 and concentrated under vacuo to afford 6-chloro-1-[2-(dimethylamino)ethyl]spiro[3,1-benzoxazine-4,4'-piperidin]-2(1H)-one as a white solid.
References:
US2007/155761,2007,A1 Location in patent:Page/Page column 15