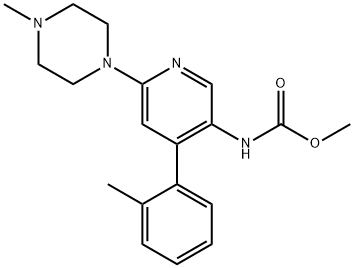
Methyl (6-(4-Methylpiperazin-1-yl)-4-(o-tolyl)pyridin-3-yl)carbaMate synthesis
- Product Name:Methyl (6-(4-Methylpiperazin-1-yl)-4-(o-tolyl)pyridin-3-yl)carbaMate
- CAS Number:342417-02-1
- Molecular formula:C19H24N4O2
- Molecular Weight:340.42
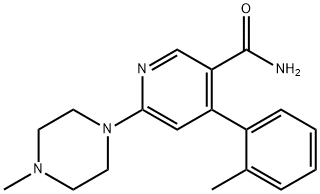
342417-01-0
157 suppliers
$5.00/100mg

124-41-4
730 suppliers
$12.00/25g

342417-02-1
18 suppliers
inquiry
Yield:342417-02-1 100%
Reaction Conditions:
Stage #1: sodium methylatewith N-Bromosuccinimide in methanol;dichloromethane at -5; for 16 h;
Stage #2: 4-(2-methylphenyl)-6-(4-methylpiperazinyl)-3-pyridinecarboxamide in methanol;dichloromethane at -5; for 5.3 h;
Steps:
1.5 Synthesis of methyl-[6-(4-methyl-piperazin-1-yl)-4-o-tolyl-pyridin-3-yl]-amine
Step 5
2.15 ml (11.6 mMol) Sodium methoxide in methanol were added over 30 minutes to a suspension of 0.85 g (4.6 mMol) N-bromosuccinimide in 5.0 ml dichloromethane cooled to -5° C.
The reaction mixture was stirred 16 hours at -5° C.
Still at this temperature, a solution of 1.0 g (3.1 mMol) 6-(4-methyl-piperazin-1-yl)-4-o-tolyl-nicotinamide in 5.0 ml methanol was added over 20 minutes and stirred for 5 hours. 7.1 ml (7.1 mMol) Aqueous HCl 1N and 20 ml dichloromethane were added.
The phases were separated and the organic phase was washed with deionized water.
The aqueous phases were extracted with dichloromethane, brought to pH=8 with aqueous NaOH 1N and further extracted with dichloromethane.
The latter organic extracts were combined, dried (Na2SO4) and concentrated to yield 1.08 g (quant.) [6-(4-methyl-piperazin-1-yl)-4-o-tolyl-pyridin-3-yl]-carbamic acid methyl ester as a grey foam. MS (ISP): 341 (M+H+, 100), 284 (35).
References:
US8426450,2013,B1 Location in patent:Page/Page column 26
5326-23-8
565 suppliers
$5.00/1g

342417-02-1
18 suppliers
inquiry
342417-00-9
6 suppliers
inquiry

342417-02-1
18 suppliers
inquiry
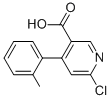
342416-99-3
7 suppliers
inquiry

342417-02-1
18 suppliers
inquiry
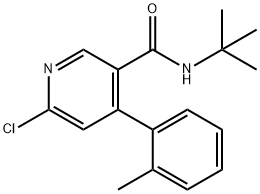
342417-04-3
49 suppliers
$15.00/250mg

342417-02-1
18 suppliers
inquiry