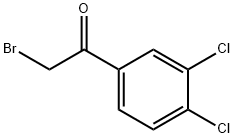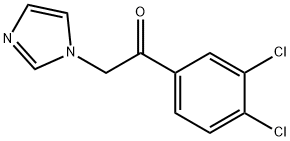
1-(3,4-DICHLOROPHENYL)-2-(1H-IMIDAZOL-1-YL)ETHANONE synthesis
- Product Name:1-(3,4-DICHLOROPHENYL)-2-(1H-IMIDAZOL-1-YL)ETHANONE
- CAS Number:37906-39-1
- Molecular formula:C11H8Cl2N2O
- Molecular Weight:255.1
Yield:37906-39-1 60%
Reaction Conditions:
in acetonitrile at 0; for 3.5 h;
Steps:
69.1 Step 1: Preparation of 1-(3,4-dichlorophenyl)-2-(1H-imidazol-1-yl)ethanone
After dissolving 1 g of 3,4-dichlorophenacyl bromide in 10 mL of acetonitrile, 762 mg of imidazole was added at 0° C., followed by stirring for 2 h and 1.5 h at rt. The solvent was removed, the residue was washed with water, extracted with MC, dried over MgSO4, and concentrated. After adding diethyl ether, it was solidified with hexane.Yield: 578 mg (60%, yellow solid)
References:
KR2021/52042,2021,A Location in patent:Paragraph 0685-0690
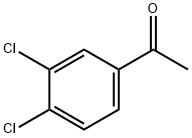
2642-63-9
190 suppliers
$6.00/5g

37906-39-1
12 suppliers
inquiry