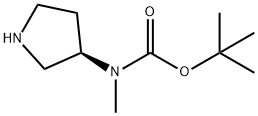
METHYL-PYRROLIDIN-3-YL-CARBAMIC ACID TERT-BUTYL ESTER synthesis
- Product Name:METHYL-PYRROLIDIN-3-YL-CARBAMIC ACID TERT-BUTYL ESTER
- CAS Number:392338-15-7
- Molecular formula:C10H20N2O2
- Molecular Weight:200.28
![benzyl (3R)-3-[(tert-butoxycarbonyl)(methyl)amino]pyrrolidine-1-carboxylate](/CAS/20180808/GIF/638217-49-9.gif)
638217-49-9

392338-15-7
General procedure for the synthesis of (R)-3-(N-BOC-N-methylamino)pyrrolidine from the compound (CAS: 638217-49-9): preparation of tert-butyl [(3R)-pyrrolidin-3-yl]carbamate; dissolution of carbamate of compound 46 (15.58 g, 46.6 mmol) in ethanol (150 mL) under 5% Pd/C (1 g) ) catalyzed hydrogenation at 50 psi hydrogen pressure and room temperature for 18 hours. Subsequently, Pd/C (500 mg) was added additionally and the hydrogenation was continued under the same conditions for 26 hours. Upon completion of the reaction, the catalyst was removed by filtration and the filtrate was concentrated under vacuum. Purification by column chromatography (eluent: DCM:MeOH:0.880 ammonia, volumetric ratio tapered from 100:0:0 to 90:10:1) afforded (R)-3-(N-BOC-N-methylamino)pyrrolidine as a light yellow oil (5.85 g, 62% yield).1H NMR (400 MHz, CDCl3) δ: 4.56 (1H, m) , 3.06 (2H, m), 2.87 (1H, m), 2.79 (1H, m), 2.78 (3H, s), 2.54 (1H, s), 1.95 (1H, m), 1.73 (1H, m), 1.43 (9H, s) ppm. m/z MS (ESI): 201 [M + H]+.
![Carbamic acid, N-methyl-N-[(3R)-1-(phenylmethyl)-3-pyrrolidinyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/762286-32-8.gif)
762286-32-8
0 suppliers
inquiry

392338-15-7
109 suppliers
$45.00/50mg
Yield:392338-15-7 100%
Reaction Conditions:
with ammonium formate;palladium 10% on activated carbon in methanol;water; for 5 h;Heating / reflux;
Steps:
5.b
(b) Title compoundA solution of the compound obtained above (14.5 g, 50.14 mmol), Pd/C (10%, 50% in water) (3g) and ammonium formate (12.7 g, 200.5 mmol) in a mixture of MeOH (390 ml_) and water (45 ml_) was heated at reflux for 5 hours. The reaction was filtered through Celite and the filtrate was washed with AcOEt and MeOH. The solvent was evaporated to dryness to afford 10.6 g of the title compound as an oil(yield: 100%).1H NMR (300 MHz, CDCI3) δ: 1.38 (s, 9H), 1.72 (m, 1 H), 1.96 (m, 1 H), 2.53 (s, NH), 2.80 (s, 3H), 2.87 (m, 1 H), 2.93 (m, 1 H), 3.11 (m, 2H), 4.58 (m, 1 H).
References:
WO2007/31529,2007,A1 Location in patent:Page/Page column 24
![benzyl (3R)-3-[(tert-butoxycarbonyl)(methyl)amino]pyrrolidine-1-carboxylate](/CAS/20180808/GIF/638217-49-9.gif)
638217-49-9
4 suppliers
inquiry

392338-15-7
109 suppliers
$45.00/50mg

24424-99-5
869 suppliers
$13.50/25G

392338-15-7
109 suppliers
$45.00/50mg
144043-17-4
58 suppliers
$45.00/100mg

392338-15-7
109 suppliers
$45.00/50mg