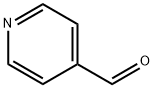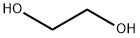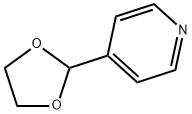
4-[1,3]Dioxolan-2-yl-pyridine synthesis
- Product Name:4-[1,3]Dioxolan-2-yl-pyridine
- CAS Number:61379-59-7
- Molecular formula:C8H9NO2
- Molecular Weight:151.16
Yield:61379-59-7 92%
Reaction Conditions:
with toluene-4-sulfonic acid in benzene;Heating / reflux;
Steps:
1
4-(1,3-Dioxolan-2-yl)pyridine A stirred solution of pyridine-4-carboxaldehyde (8.7 ml, 90 mmol), ethylene glycol (10 ml, 180 mmol) and p-toluene sulfonic acid (18.8 g, 99 mmol) in benzene (70 ml) was refluxed overnight. A Dean-Stark apparatus was used to remove water azeotropically from the reaction. After-15 h the mixture was cooled, then made basic with aq. NaOH (20 % w/v,-30 ml). The benzene layer was isolated and the aqueous layer was washed with dichloromethane until no more product came out (5 x 60 ml). The combined organic phases were dried (Na2SO4) and solvent removed in vacuo to give the pure 4- (1, 3 dioxolan-2-yl) pyridine [Registry No. 61379-59-7] as a pale yellow liquid that solidified under vacuum (12.57 g, 92 %).'H NMR (300 MHz, CDCI3) : s 4.06 (4H, m, 0- (CH2) 2-0), 5.82 (1H, s, O-CH-O), 7.39 (2H, d, J= 6 Hz, H-3, H-5), 8.63 (2H, d, J= 6 Hz, H-2, H-6) ppm.
References:
WO2005/46330,2005,A1 Location in patent:Page/Page column 18-19
646-06-0
353 suppliers
$15.00/25mL
110-86-1
888 suppliers
$9.00/5g
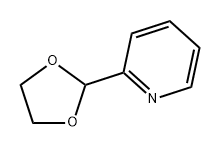
5693-54-9
9 suppliers
inquiry
![4-[1,3]Dioxolan-2-yl-pyridine](/CAS/20180529/GIF/61379-59-7.gif)
61379-59-7
6 suppliers
inquiry

96517-55-4
1 suppliers
inquiry
96517-54-3
1 suppliers
inquiry